Overview
Combined hormonal contraception (CHC) is a popular choice of contraception around the world, with over a third of UK women using oral contraception. Data suggest that most women on oral contraception opt for combined oral contraception (COC). CHC uses oestrogen and progestin to prevent pregnancies. The following Guidelines summary includes recommendations on:
- the use of CHCs
- the effectiveness of CHC
- non-contraceptive health benefits
- health risks
- information on CHC consultations
- CHC follow-up
- giving specific advice to those on CHC
- stopping CHC
- use in perimenopause.
How Is CHC Used?
Key Information
- Tailored CHC regimens can reduce the frequency of withdrawal bleeds and can reduce withdrawal symptoms associated with the hormone-free interval; however, unscheduled bleeding is common.
Clinical Recommendations
- Women should be given information about both standard and tailored CHC regimens to broaden contraceptive choice
- Women should be advised that use of tailored CHC regimens is outside the manufacturer’s licence but is supported by the Faculty of Sexual and Reproductive Healthcare (FSRH)
- Women should have access to clear information (either written or digital) to support tailored CHC use.
When Can CHC Be Started?
Key Information
- CHC containing ethinyloestradiol (EO) can be started by medically eligible women up to and including day 5 of a natural menstrual cycle without the need for additional contraceptive protection
- CHC containing EO can be quick-started by medically eligible women at any other time (with advice to use additional contraceptive precaution for 7 days) if:
- it is reasonably certain that the woman is not pregnant or
- a high-sensitivity urine pregnancy test is negative (even if there is a risk of pregnancy from unprotected sexual intercourse [UPSI] in the last 21 days). A follow-up high-sensitivity urine pregnancy test is required 21 days after the last UPSI.
How Effective Is CHC?
Contraceptive Effectiveness of CHC
Key Information
- Contraceptive effectiveness of all CHCs is similar
- If used perfectly, CHC is very effective for contraception. With typical use, CHC is less effective for contraception than long-acting reversible contraception (LARC).
Clinical Recommendations
- Women requesting CHC should be informed about the effectiveness (with both typical and perfect use) of CHC and other contraceptive methods, including the superior effectiveness of LARC.
Is Contraceptive Effectiveness of CHC Affected by Obesity/Weight?
Key Information
- Most evidence suggests no association between weight/body mass index (BMI) and effectiveness of COCs
- Limited evidence suggests a possible reduction in patch effectiveness in women weighing 90 kg or more.
Is Contraceptive Effectiveness of CHC Affected by Bariatric Surgery?
Clinical Recommendation
- Women who have had bariatric surgery should be advised that the effectiveness of COC could be reduced.
What Drug Interactions Are Important to Consider in Relation to CHC?
Enzyme-inducing Drugs
Clinical Recommendations
- Women using enzyme-inducing drugs should be informed that the contraceptive effectiveness of CHC could be reduced during use of the enzyme-inducer and for 28 days after stopping
- Women using enzyme-inducing drugs should be offered a reliable contraceptive method that is unaffected by enzyme-inducers.
Lamotrigine
Clinical Recommendation
- Women taking lamotrigine should be advised that CHC may interact with lamotrigine; this could result in reduced seizure control or lamotrigine toxicity. The risks of using CHC could outweigh the benefits.
Antibiotics (Non Enzyme-Inducing)
Key Information
- Additional contraceptive precautions are not required when antibiotics that do not induce enzymes are used in conjunction with CHCs.
Progestogen Receptor Modulators
Clinical Recommendation
- Women should be advised to wait 5 days after taking ulipristal acetate for emergency contraception before starting CHC. Women should be made aware that they must use condoms reliably or abstain from sex during the 5 days waiting and then until their contraceptive method is effective.
Severe Diarrhoea or Vomiting
Clinical Recommendation
- Women using COC should be advised that contraceptive effectiveness could be reduced by vomiting or severe diarrhoea.
Incorrect Use of CHC
- See FSRH Guidance on Incorrect use of combined hormonal contraception.
Non-contraceptive Health Benefits Associated With CHC Use
Key Information
- Use of CHC can reduce heavy menstrual bleeding and menstrual pain, and improve acne
- Use of CHC may be beneficial for women with premenstrual syndrome symptoms
- Use of CHC (particularly continuous CHC regimens) can reduce the risk of recurrence of endometriosis after surgical management
- CHC can be used for the management of acne, hirsutism, and menstrual irregularities associated with polycystic ovary syndrome
- CHC use is associated with a significant reduction in the risk of endometrial and ovarian cancer that increases with duration of CHC use and persists for many years after stopping CHC
- Use of CHC is associated with a reduced risk of colorectal cancer.
Health Risks Associated With CHC Use
Clinical Recommendation
- Women should be informed about the health risks associated with use of CHC.
Venous Thromboembolism (Including Deep Vein Thrombosis and Pulmonary Embolism)
Key Information
- Current use of CHC is associated with an increased risk of venous thromboembolism (VTE); some CHC formulations are associated with a greater risk of VTE than others, dependent on progestogen type and oestrogen dose.
Clinical Recommendation
- Women should be advised that the use of CHC is associated with an increased risk of VTE, but the absolute risk of VTE for an individual CHC user remains very small.
Arterial Thromboembolic Disease
Key Information
- Current use of CHC is associated with a very small increased risk of myocardial infarction (MI) and ischaemic stroke that appears to be greater with higher doses of oestrogen in COC.
Clinical Recommendation
- Women should be informed that current use of CHC is associated with an increased risk of MI and ischaemic stroke but that that these events are still extremely uncommon in CHC users
- Use of CHC by women with significant additional risk factors for arterial disease should be strongly cautioned or avoided.
Breast Cancer
Clinical Recommendation
- Women should be advised that current use of CHC is associated with a small increased risk of breast cancer, which reduces with time after stopping CHC.
Cervical Cancer
Clinical Recommendation
- Women should be advised that current use of CHC for more than 5 years is associated with a small increased risk of cervical cancer; risk reduces over time after stopping CHC and is no longer increased by about 10 years after stopping.
What Should Be Done in an Initial CHC Consultation?
- Algorithm 1 summarises the suggested content of an initial consultation with a woman who requests CHC. The consultation should focus on provision of safe, effective contraception that suits a woman’s requirements and that could also provide non-contraceptive benefits. Such a consultation is, however, an important opportunity to assess whether a woman is already at risk of pregnancy and could require emergency contraception or pregnancy testing, to assess STI risk and offer advice and testing, and to remind women about the importance of cervical screening.
Algorithm 1: Suggested Content of an Initial CHC Consultation
Please see the full guideline for further details on the topics given in Algorithm 1.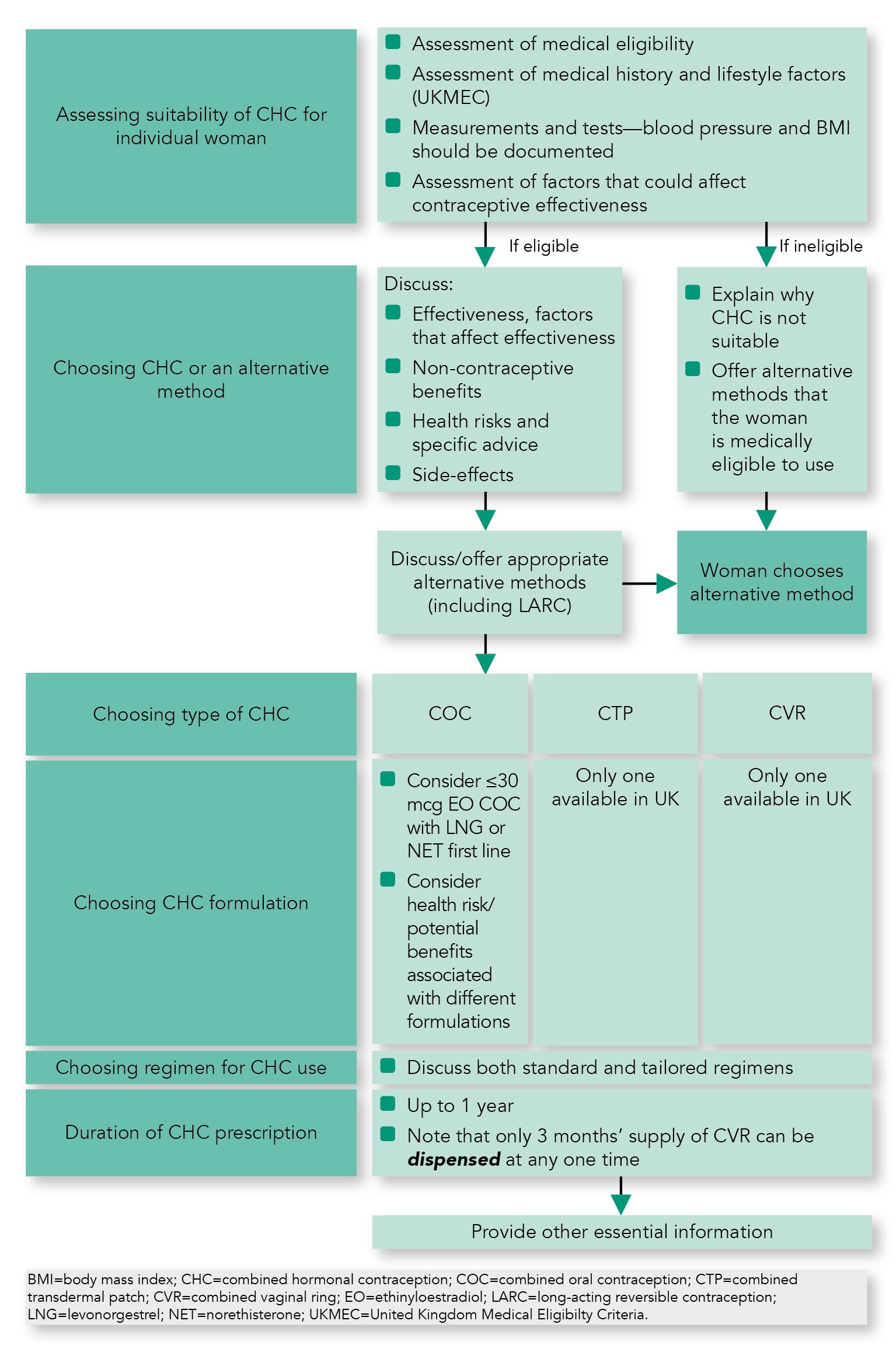
Assessment of Suitability of CHC for an Individual Woman
Key Information
- Use of suitable self-completed checklists for medical eligibility appears to be accurate and acceptable to women.
Clinical Recommendations
- Assessment of medical eligibility for CHC should include medical conditions, lifestyle factors, and family medical history
- A drug history should identify:
- any prescribed or non-prescribed drug that could affect the effectiveness of the contraceptive
- any prescribed or non-prescribed drug that could itself be affected by the contraceptive
- A recent, accurate blood pressure recording should be documented for all women prior to first CHC prescription
- BMI should be documented for all women prior to CHC prescription
- Pelvic examination is not required prior to initiation of CHC
- Breast examination, cervical screening, testing for thrombophilia, hyperlipidaemia or diabetes mellitus, and liver function tests are not routinely required prior to initiation of CHC
- Women for whom CHC is unsuitable should be offered alternative effective contraception.
Choosing Type and Formulation of CHC
Key Information
- COC containing 30 mcg or greater EO in combination with levonorgestrel or norethisterone is a reasonable first-line choice of CHC to minimise cardiovascular risk.
Other Important Supporting Information
Clinical Recommendation
- Women should be provided with written information or a link to a trusted online resource to support safe, effective CHC use.
Duration of CHC Prescription
Clinical Recommendation
- The healthcare practitioner can prescribe up to 12 months’ supply of CHC for women who are initiating or continuing CHC.
What Follow Up Is Required for Women Continuing With Use of CHC?
What Follow-up Arrangements Are Appropriate?
Clinical Recommendation
- Women should be advised that routine annual review of their contraception is recommended during CHC use.
What Should Be Done at CHC Follow Up?
Clinical Recommendation
- Medical eligibility, drug history, method adherence, and method satisfaction should be reassessed at follow up. BMI and blood pressure should be recorded.
What Specific Advice Is Required for Women Using CHC?
CHC Use During Travel
Clinical Recommendation
- Women using CHC should be advised about reducing periods of immobility during travel.
CHC Use at High Altitude
Clinical Recommendation
- Women trekking to high altitudes (above 4500 m or 14,500 ft) for periods of more than 1 week may be advised to consider switching to a safer alternative contraceptive method.
Surgery/Periods of Immobilisation
Clinical Recommendation
- Women should be advised to stop CHC and to switch to an alternative contraceptive method at least 4 weeks prior to planned major surgery or expected period of limited mobility.
What Recommendations Are There Regarding Stopping CHC?
How Long Can Women Use CHC?
Clinical Recommendation
- CHC can be used by medically eligible women for contraception until age 50 years.
Use of CHC in Perimenopause
Use of CHC as an Alternative to Hormone Replacement Therapy
Clinical Recommendation
- CHC can be considered for use by medically eligible women until age 50 years as an alternative to hormone replacement therapy for relief of menopausal symptoms and prevention of loss of bone mineral density, as well as for contraception.

