Overview
This Guidelines summary covers the assessment, diagnosis, and current treatments for osteoporosis, including recommendations to prevent fragility fractures. It applies to postmenopausal women and men aged 50 years or older.
This summary only covers key recommendations for primary care. For a complete set of recommendations, refer to the full guideline.
There are two strengths of recommendation made in this guideline, informed by the balance between desirable and undesirable effects, the quality of the evidence base, values and preferences, and resource allocation within the UK health community. For more information on the wording used, including strong and conditional recommendations, refer to the full guideline.
Key:
[✓] Strong recommendation
[C] Conditional recommendation
Reflecting on Your Learnings
Reflection is important for continuous learning and development, and a critical part of the revalidation process for UK healthcare professionals. Click here to access the Guidelines Reflection Record.
Fracture Risk Assessment and Case Finding
- A Fracture Risk Assessment Tool (FRAX®) assessment should be performed in any postmenopausal woman, or man aged 50 years or older, with a clinical risk factor for fragility fracture, to guide bone mineral density (BMD) measurement and prompt timely referral and/or drug treatment, where indicated [✓]
- When using FRAX® to calculate the probability of fracture, clinical judgement is needed when clinical risk exceeds those factors able to be entered into FRAX® [✓]
- Arithmetic adjustments to FRAX® probabilities of major osteoporotic fracture (MOF: clinical spine, hip, forearm, or humerus) and hip fracture (see Table 2 in the full guideline) can be used in clinical practice, to take account of additional clinical risk factors, such as glucocorticoid use, discordantly low lumbar spine BMD, type 2 diabetes, and a history of falls [C]
- Vertebral fracture assessment (VFA) is indicated in postmenopausal women and men aged 50 years or older, if there is a history of 4 cm or greater height loss, kyphosis, recent or current long-term oral glucocorticoid therapy, a BMD T-score of -2.5 or under at either the spine or hip, or in cases of acute onset back pain with risk factors for osteoporosis [✓]
- T-scores in men and women derived from femoral neck BMD should use normative values for BMD derived from young healthy women from the third National Health and Nutrition Examination Survey (NHANES III) [✓]
- Dual-energy X-ray absorptiometry (DXA) scan results should be reported within 3 weeks of the scan, by healthcare professionals with specific training in DXA interpretation, and in accordance with national and international reporting standards [✓]
- Patients with osteoporosis and/or a fragility fracture should be investigated for underlying causes; this includes the need for routine blood tests [✓]
- The use of quantitative ultrasound is not recommended for the diagnosis of osteoporosis [✓]
- Quantitative computed tomography (QCT)-measured femoral neck areal BMD in postmenopausal women and men aged 50 years or older can be used for opportunistic diagnosis of osteoporosis and to inform individual treatment decisions using FRAX® [C]
- Computer-aided diagnostics (CAD) may be considered to improve standard reporting of computed tomography scans (CTs) performed on postmenopausal women and men aged 50 years or older to improve opportunistic identification of vertebral fractures [C].
Measurement of Bone Mineral Density
- The risk of fracture increases progressively with decreasing BMD
- The World Health Organization and the International Osteoporosis Foundation recommend that the reference technology for the measurement of BMD is DXA applied to the femoral neck, because of its higher predictive value for fracture (evidence level Ia). DXA measurements of femoral neck BMD are used in FRAX®
- The spine is not always a reliable site for risk assessment or for the diagnosis of osteoporosis in older people because of the high prevalence of degenerative changes, which artefactually increase the BMD value. However, a result in an older person showing low BMD is almost always valid and clinically useful, particularly in those people with disproportionately low spine BMD compared to the hip
- Osteoporosis can be diagnosed on the basis of the BMD T-score measured at the total hip, femoral neck, or lumbar spine. However, fracture risk prediction is not improved by the use of measurements from multiple sites (evidence level IIa)
- Where hip BMD measurement is not possible for technical reasons, or if the spine is differentially affected, then spine BMD measurements can be used for diagnosis
- A diagnosis of osteoporosis can be made based on distal forearm (1/3 radius) T-Score if neither spine nor hip can be reliably measured or interpreted, or if a patient exceeds the weight limit for the DXA table (evidence level IV)
- Serial BMD measurement can be used to monitor response to treatment (see Section 7 of the full guideline). Lumbar spine BMD shows the largest treatment-related changes and is the preferred site, although if spinal degenerative changes are marked, BMD at the hip is a better site for monitoring.
Fracture Risk Assessment Tools
- The FRAX® tool (shef.ac.uk/FRAX) computes the 10-year probability of hip fracture and/or of major osteoporotic fracture. A major osteoporotic fracture is a clinical spine, hip, forearm, or humerus fracture. The tool has been externally validated in independent cohorts (evidence level Ia)
- QFracture (qfracture.org) is based on a UK prospective open cohort study of routinely collected data from general practices that takes into account numerous clinical risk factors, and estimates the 1–10-year cumulative incidence of hip and/or major osteoporotic fracture
- NICE has recommended the use of fracture risk assessment tools (FRAX® or QFracture) in the assessment of patients. Since FRAX® and QFracture yield different outputs (probability of fracture accounting for mortality risk in the case of FRAX®, and a cumulative risk of fracture in the case of QFracture), the two calculators cannot be used interchangeably
- BMD cannot be incorporated into QFracture estimations
- National Osteoporosis Guideline Group (NOGG) intervention thresholds, recommended by NICE Quality Standards, are based on FRAX® probability and thus cannot be used with fracture risk derived from QFracture or other calculators
- The input into FRAX® includes, with age and sex, the BMD-independent clinical risk factors listed in Box 1. Femoral neck BMD is an optional input. The listed secondary causes are conservatively assumed to be mediated through low BMD and carry no weight when femoral neck BMD is entered into FRAX®.
| Box 1: Clinical Risk Factors Included Specifically in the FRAX® Assessment of Fracture Probability |
|---|
|
NB Additional clinical risk factors that should prompt FRAX® assessment are listed below in Box 2.
Adjustments to FRAX probabilities which take into account severity and/or number of vertebral fractures cannot currently be made because of the lack of appropriate empirical data.
For recommendations on FRAX® adjustments and considerations to aid the interpretation of FRAX®, refer to the full guideline.
| Box 2: Clinical Risk Factors for Osteoporosis/Fractures not Accommodated in FRAX®, Which Should Trigger Risk Assessment |
|---|
Abbreviations: FRAX®=Fracture Risk Assessment Tool; TSH=thyroid-stimulating hormone |
Investigation of Osteoporosis and Fragility Fractures
- Diagnostic assessment of individuals with osteoporosis should exclude diseases that mimic osteoporosis, identify the cause(s) of the osteoporosis, and include the management of any associated comorbidity. Common investigations are given in Table 1.
Table 1: Proposed Clinical Investigations to Consider for the Investigation of Osteoporosis/Fragility Fractures
| Routine | Other Procedures, If Indicated |
|---|---|
| Abbreviations: BMD=bone mineral density; CTX=serum cross-linked C-telopeptide of type I collagen; DXA=dual-energy X-ray absorptiometry; FRAX®=Fracture Risk Assessment Tool; P1NP=procollagen type 1 N-terminal propeptide; PTH=parathyroid hormone | |
| Clinical history Physical examination, including measurement of height and assessment of thoracic kyphosis Full blood cell count Erythrocyte sedimentation rate or C-reactive protein Serum calcium, albumin, creatinine, phosphate[A] , alkaline phosphatase[A] , and liver transaminases Serum 25-hydroxyvitamin D Thyroid function tests | Serum electrophoresis, serum immunoglobulins, and serum free light chain assay Plasma PTH[B] Serum testosterone, sex hormone binding globulin, follicle stimulating hormone, luteinising hormone 24-hour urinary free cortisol/overnight dexamethasone suppression test Serum prolactin Serum magnesium if hypocalcaemic Tissue transglutaminase antibodies, +/- endomysial antibodies (coeliac disease screen) Urinary calcium excretion Markers of bone turnover (e.g. CTX, P1NP)[C] Lateral radiographs of lumbar and thoracic spine or DXA-based lateral vertebral imaging DXA if indicated by FRAX® assessment and/or required for BMD monitoring Isotope bone scan |
| [A] Persistent low phosphate or alkaline phosphatase should not be overlooked, as this can indicate underlying metabolic bone disease [B] Measure PTH if albumin-adjusted serum calcium ≥ 2.6mmol/l twice, or if ≥ 2.5mmol/l twice if primary hyperparathyroidism is suspected [C] Principally measured to monitor bone turnover in response to anti-resorptive treatment (see Section 7 of the full guideline); CTX reflects bone resorption, P1NP bone formation. CTX is best measured in the morning after an overnight fast Other investigations, for example, bone biopsy and genetic testing for osteogenesis imperfecta, are largely restricted to specialist centres. | |
For recommendations on vertebral fracture assessment, and screening and case finding, refer to the full guideline.
Intervention Thresholds and Strategy
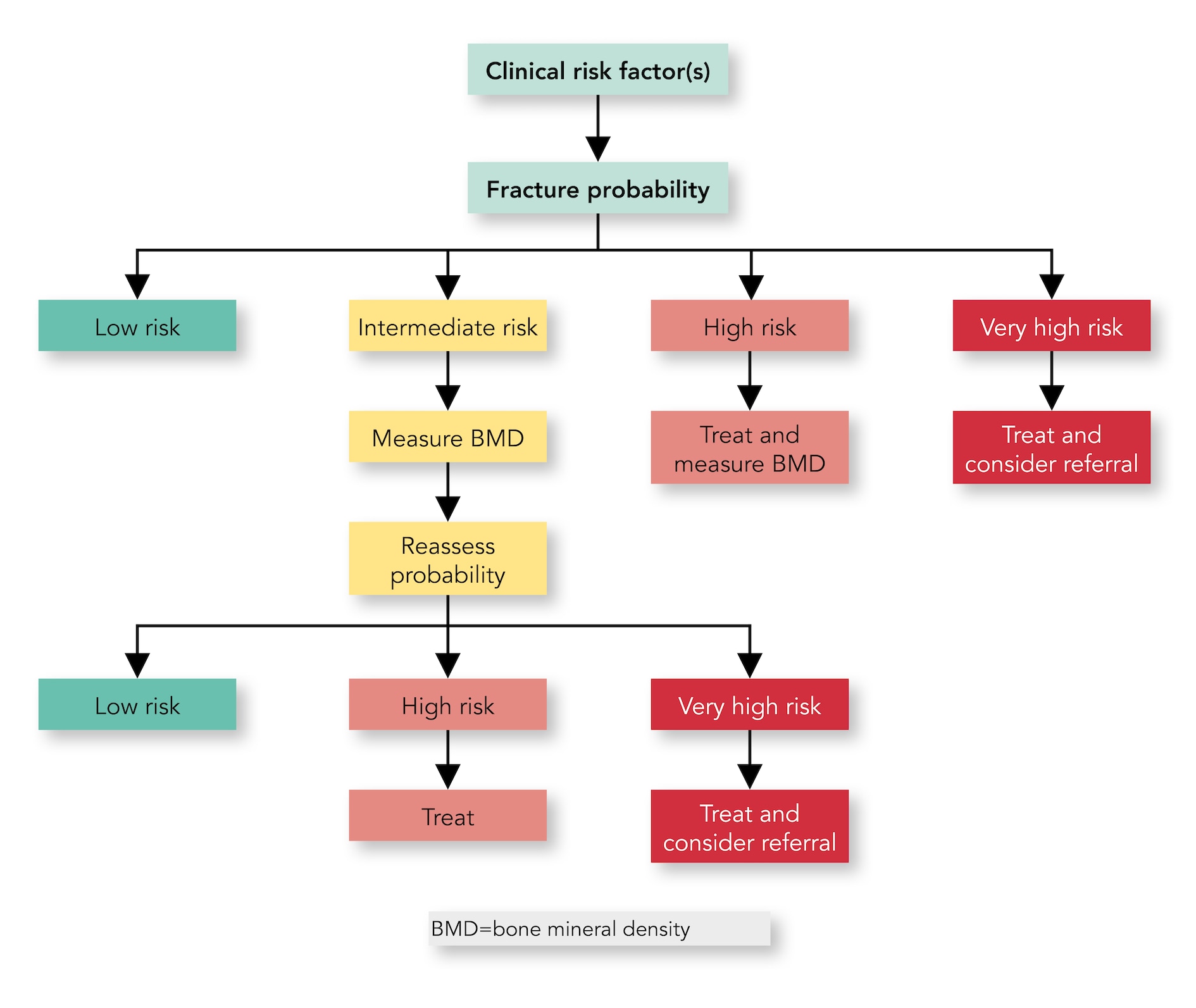
- An initial FRAX® assessment, which provides the 10-year probability of a major MOF (clinical spine, hip, forearm, or humerus) and/or hip fracture, can be used to identify patients at low, intermediate, high, or very high risk of fracture [✓]
- Consider, particularly in older people, drug treatment in those with a prior and/or recent fragility fracture, with fracture risk assessment informing the choice of drug treatment [✓]
- Men and women with high and very high fracture risk (see Algorithm 1) should have a DXA if a baseline measurement is needed against which to compare future BMD measurements [✓]
- Men and women with intermediate fracture risk (that is, between the upper and lower assessment thresholds) should be referred for BMD measurement, if practical. Thereafter, fracture probability should be reassessed using FRAX® [✓]
- When BMD is included in a FRAX® assessment, the patient’s risk (high, very high, or low) is determined by the higher of the two (MOF and hip fracture) risk assessments [✓]
- In men and women with intermediate fracture risk, if BMD measurement is unavailable, contraindicated, or impractical (for example, in frail individuals), drug treatment should be offered if there is a history of fragility fracture and/or if fracture risk exceeds the intervention threshold (which is shown in Algorithm 1) [✓]
- Men and women with low fracture risk, without a prior fragility fracture, can be reassured that their fracture risk is low and offered lifestyle advice as appropriate [✓]
- Consider referral of very high-risk patients to an osteoporosis specialist in secondary care for assessment and consideration of parenteral treatment (some may need first-line anabolic drug treatment, especially those with multiple vertebral fractures). Indications for specialist referral include [C]:
- the presence of single but important clinical risk factors, such as
- a recent vertebral fracture (within the last 2 years)
- two or more vertebral fractures (whenever they have occurred)
- BMD T-Score of -3.5 or under
- treatment with high-dose glucocorticoids (7.5 mg/day or more of prednisolone, or equivalent, over 3 months). Refer urgently given rapid loss in bone post initiation of glucocorticoids; if any delay is anticipated, start an oral bisphosphonate in the meantime
- the presence of multiple clinical risk factors, particularly with a recent fragility fracture indicating high imminent risk of re-fracture, or
- other indicators of very high fracture risk
- the presence of single but important clinical risk factors, such as
- The choice of drug treatment should be informed by the level of fracture risk, additional clinical risk factors, cost-effectiveness of treatment, and patient preferences [✓]
- FRAX® and the link to the NOGG website should be incorporated into electronic patient health record systems [✓].
FRAX® Assessment Thresholds for 10-year Probability of Fracture
- The approach recommended for decision-making is based on fracture probabilities derived from FRAX® and can be applied to men and women. This approach is underpinned by cost-effectiveness analysis, with oral or intravenous bisphosphonates as the intervention (evidence level Ib)
- FRAX® assessment thresholds for 10-year probability of a MOF are shown in Algorithm 1.
Algorithm 1: NOGG Assessment, Intervention, and Risk Thresholds for MOF Probability in the UK With the Use of FRAX®
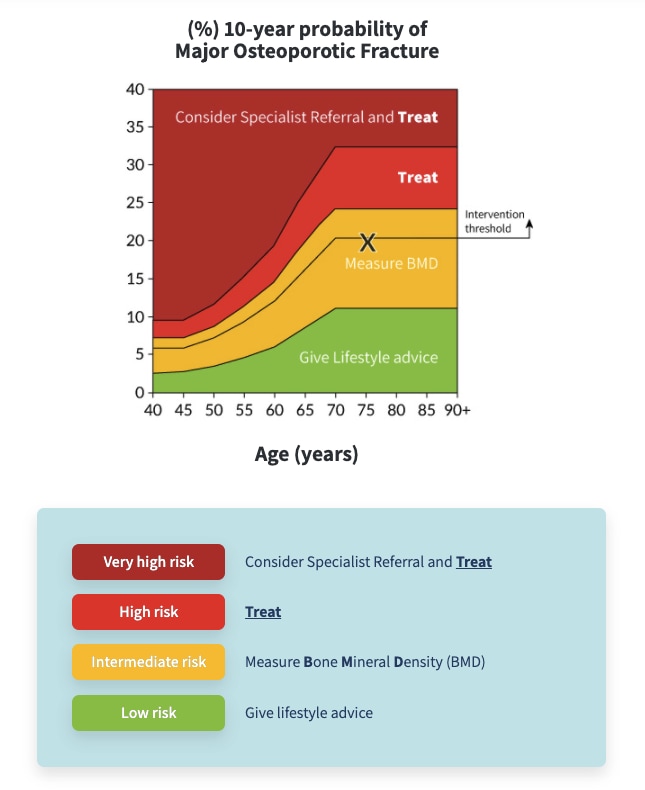
Low risk: give lifestyle advice, and reassess in 5 years or less depending on the clinical context.
Intermediate risk: measure BMD and recalculate fracture risk. If BMD measurement not practical (e.g. frailty) or is unavailable, offer treatment if risk is at, or above, the intervention threshold shown.
High risk: offer treatment to reduce fracture risk. Measure BMD to guide drug choice and provide a baseline for BMD monitoring.
Very high risk: Consider referral to osteoporosis specialist for assessment and consideration of parenteral treatment. If a delay anticipated, start oral treatment in meantime. If not for referral, treat as for high risk.
NB these thresholds are for guidance only and the final decision to assess BMD or to initiate therapeutic intervention lies with the individual clinician.
Abbreviations: BMD=bone mineral density; MOF=major osteoporotic fracture
- The use of FRAX® without BMD has approximately the same performance as BMD without FRAX® (evidence level Ia). Thus, the same intervention threshold can be used when fracture risk is assessed with or without BMD (see Algorithm 1)
- For men and women, the intervention threshold up to age 70 years is set at a risk equivalent to that of a woman of the same age with a prior fracture, in line with current clinical practice, and therefore rises with age. At age 70 years and above, fixed thresholds are applied (evidence level Ib)
- The proportion of women potentially eligible for treatment rises from approximately 30% to 50% with age, largely driven by the prevalence of prior fracture (evidence level Ib)
- When FRAX® is calculated with BMD included, the NOGG website also provides intervention thresholds based on the 10-year probability of hip fracture, in addition to the 10-year probability of a MOF (see Figure 2 in the full guideline). If there is discordance between the risk categories identified by the two probabilities, the highest risk category can be used to guide intervention.
Indications for Specialist Referral in Those at Very High Fracture Risk
- Individuals at very high fracture risk have the most to gain from thorough investigation of osteoporosis, falls assessment, and development and delivery of a personalised treatment plan for a chronic, life-long condition. A number of treatments now available to treat osteoporosis are mostly (but not exclusively) initiated through secondary care (see the section, Pharmacological treatment options)
- Indications for referral to an osteoporosis specialist may arise through several routes, for example, in the presence of single but important clinical risk factors, such as a recent vertebral fracture (within the last 2 years), two or more vertebral fractures (whenever they have occurred), a BMD T-Score of -3.5 or under, high-dose glucocorticoids use (7.5 mg/day or more of prednisolone, or equivalent, over 3 months; see Section 7 of the full guideline) (evidence levels IIb and IV), or via a combination of clinical risk factors, resulting in very high fracture risk (evidence level IIb)
- Prior fragility fracture is a well-established risk factor for a future fracture. This risk of subsequent osteoporotic fracture is particularly acute immediately after an index fracture and wanes progressively over the next 2 years, but thereafter remains higher than that of the general population. This effect of recency of fracture, sometimes termed imminent risk, is also dependent on age, sex, and site of fracture (evidence level Ic)
- A NOGG threshold that characterises men and women at high and very high fracture risk has also been established using FRAX® probabilities; very high risk is identified as a FRAX®-based fracture probability that exceeds the intervention threshold by 60% (see Algorithm 1 and Algorithm 2). It can be used to identify patients who likely require specialist referral for assessment of their osteoporosis (which should include DXA measurement of BMD), and further consideration of appropriate treatment strategies. The proportion of postmenopausal women at very high risk defined in this way rises from approximately 6% at age 50–54 to 36% at age 90 years or older. Numerical values for the probability thresholds are given in Table 5 of the full guideline for MOF and for hip fracture. An assessment algorithm is shown in Algorithm 3
- In patients with FRAX® probabilities in the high-risk category, consideration of additional clinical risk factors (for example, frequent falls, very low spine BMD—see Table 2 in the full guideline) can also lead to redesignation from high to very high risk of fracture.
Algorithm 2: NOGG Thresholds for Intervention and/or Referral Using MOF and Hip Fracture Probabilities in the UK
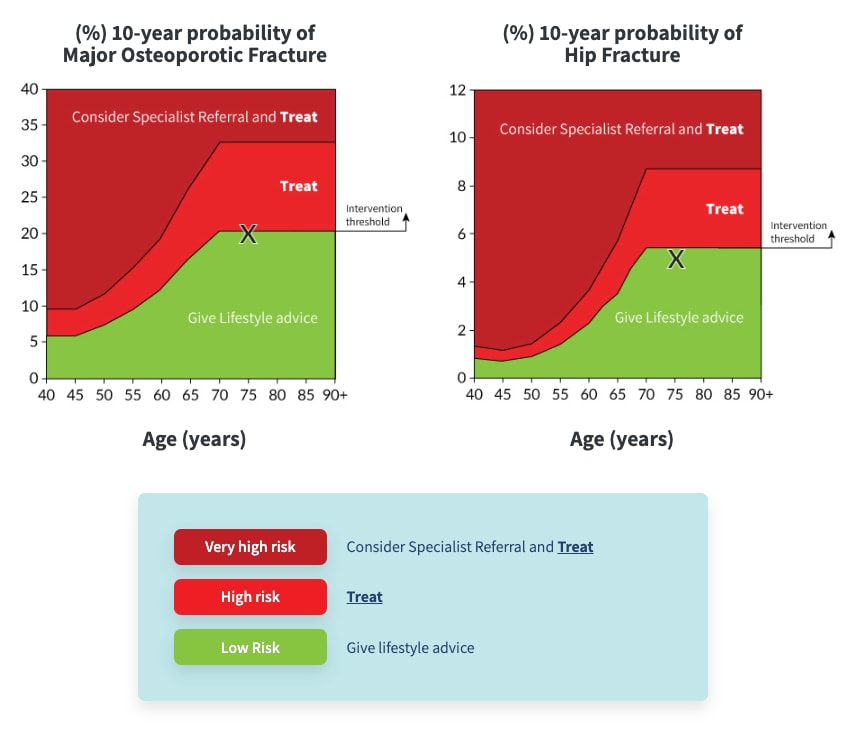
Where both the MOF and hip fracture probabilities fall below the treatment threshold, lifestyle advice should be given and a further assessment is recommended in 5 years or less depending on the clinical context (e.g. new clinical risk factors develop, or a fragility fracture occurs).
Low risk: give lifestyle advice, and reassess in 5 years or less depending on the clinical context.
High risk: offer treatment to reduce fracture risk.
Very high risk: Consider referral to osteoporosis specialist for assessment and consideration of parenteral treatment. If a delay anticipated, start oral treatment in meantime. If not for referral, treat as for high risk.
NB these thresholds are for guidance only and the final decision to assess BMD or to initiate therapeutic intervention lies with the individual clinician.
Abbreviations: BMD=bone mineral density; MOF=major osteoporotic fracture
Algorithm 3: Management Algorithm for the Assessment of Individuals at Risk of Fracture
FRAX®—Practical Considerations
- The FRAX® MOF probabilities are transferred automatically to the NOGG website by clicking on the specified button on the FRAX® results box. Where practitioners receive the results of a FRAX® risk assessment for an individual patient without treatment guidance, the FRAX® probabilities can also be entered manually onto the NOGG website (nogg.org.uk/manual-data-entry); this page also captures additional information (age, sex, glucocorticoid exposure, and whether a femoral neck BMD has been included in the FRAX® estimates) so that the result can be automatically compared to the NOGG thresholds with appropriate guidance on treatment
- Lack of integration of FRAX® assessments and links to NOGG guidance in existing patient health record systems represents a barrier to effective fracture risk assessment (evidence IV)
- FRAX® is not recommended as a tool to monitor treatment (evidence level IIb). However, the use of FRAX® is appropriate to re-evaluate current fracture probabilities when considering a change in patient management (evidence level IV).
Non-pharmacological Management of Osteoporosis
- Postmenopausal women, and men aged 50 years or older, with osteoporosis or who are at risk of fragility fracture are recommended:
- healthy, nutrient-rich, balanced diet [✓]
- an adequate intake of calcium (minimum 700 mg daily), preferably achieved through dietary intake or otherwise by supplementation [✓]
- to consume vitamin D from foods and be prescribed vitamin D supplements of at least 800 IU/day if they have identified vitamin D insufficiency or risk factors for vitamin D insufficiency. Those who are either housebound or living in residential or nursing care are more likely to require calcium and vitamin D supplementation to achieve recommended levels of intake [✓]
- a combination of regular weight-bearing and muscle-strengthening exercise, tailored according to the individual patient’s needs and ability [✓]
- advice about smoking cessation if an individual is a smoker [✓]
- advice to restrict alcohol intake to two units/day or under [✓]
- a falls assessment should be undertaken in all patients with osteoporosis and fragility fractures; those at risk should be offered exercise programmes to improve balance and/or that contain a combined exercise protocol [✓].
Pharmacological Treatment Options
- Fracture risk assessment, patient suitability and preference, and cost-effectiveness should inform the choice of drug treatment. In most people at risk of fragility fracture, anti-resorptive therapy is the first-line option [✓]
Antiresorptive Drug Treatment
- Offer oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate as the most cost-effective interventions. Alternative options include denosumab, ibandronate, hormone replacement therapy (HRT), raloxifene, and strontium ranelate [✓]
- Offer intravenous zoledronate as a first-line treatment option following a hip fracture [✓]
- Before starting denosumab, ensure a long-term personalised osteoporosis management plan is in place and that both the patient and the primary care practitioner are made aware that denosumab treatment should not be stopped or delayed without discussion with a healthcare professional [✓]
- Avoid unplanned cessation of denosumab because it can lead to increased vertebral fracture risk, hence it must not be stopped without considering an alternative therapy [✓]
- If denosumab therapy is stopped, intravenous infusion of zoledronate is recommended 6 months after the last injection of denosumab, with subsequent monitoring of serum cross-linked C-telopeptide of type I collagen (CTX) guiding the timing of further treatment [✓]
- Where monitoring of serum CTX is not possible, consider a further intravenous infusion of zoledronate 6 months after the first dose of zoledronate [C]
- Limit the initiation of HRT for the treatment of postmenopausal osteoporosis to younger post-menopausal women (aged 60 years or under) who have low baseline risk for adverse malignant and thromboembolic events [✓]
- Discuss continued use of HRT after the age of 60 years with the patient, with treatment based on an individual risk-benefit analysis [C].
Anabolic Drug Treatment
- Consider teriparatide or romosozumab as first-line treatment options in postmenopausal women at very high fracture risk, particularly in those with vertebral fractures (see the section, Intervention thresholds and strategy) [C]
- Consider teriparatide as a first-line treatment option in men aged 50 years and older who are at very high fracture risk, particularly in those with vertebral fractures (see the section, Intervention thresholds and strategy) [C]
- Consider as second-line treatment options teriparatide in postmenopausal women and men aged 50 years and older, and romosozumab in postmenopausal women who are intolerant of bisphosphonate treatment, particularly in those with vertebral fractures [C]
- Following the approved duration of treatment with teriparatide or romosozumab (24 months or 12 months respectively), initiate treatment with alendronate, zoledronate, or denosumab without delay [✓]
- Consider raloxifene as an option for follow-on treatment after an anabolic drug in women [C].
Overview of Treatment Options
- Drugs used in the management of osteoporosis can be considered under two broad headings based on their primary mode of action:
- anti-resorptive drugs primarily inhibit osteoclastic bone resorption, with later secondary effects on bone formation
- anabolic drugs primarily stimulate osteoblastic bone formation, with variable effects on bone resorption
- Most drugs fit into one category or the other, but romosozumab has a dual action, both stimulating bone formation and inhibiting bone resorption
- Anti-resorptive drugs are much less expensive than anabolic drugs
- It is important to consider the long-term management strategy for each patient initiated on osteoporosis treatment, as the timing of use of certain drugs is important; for example, teriparatide can only be used once in a lifetime, whilst denosumab requires careful consideration before initiation given the difficulties in stopping treatment once it is started.
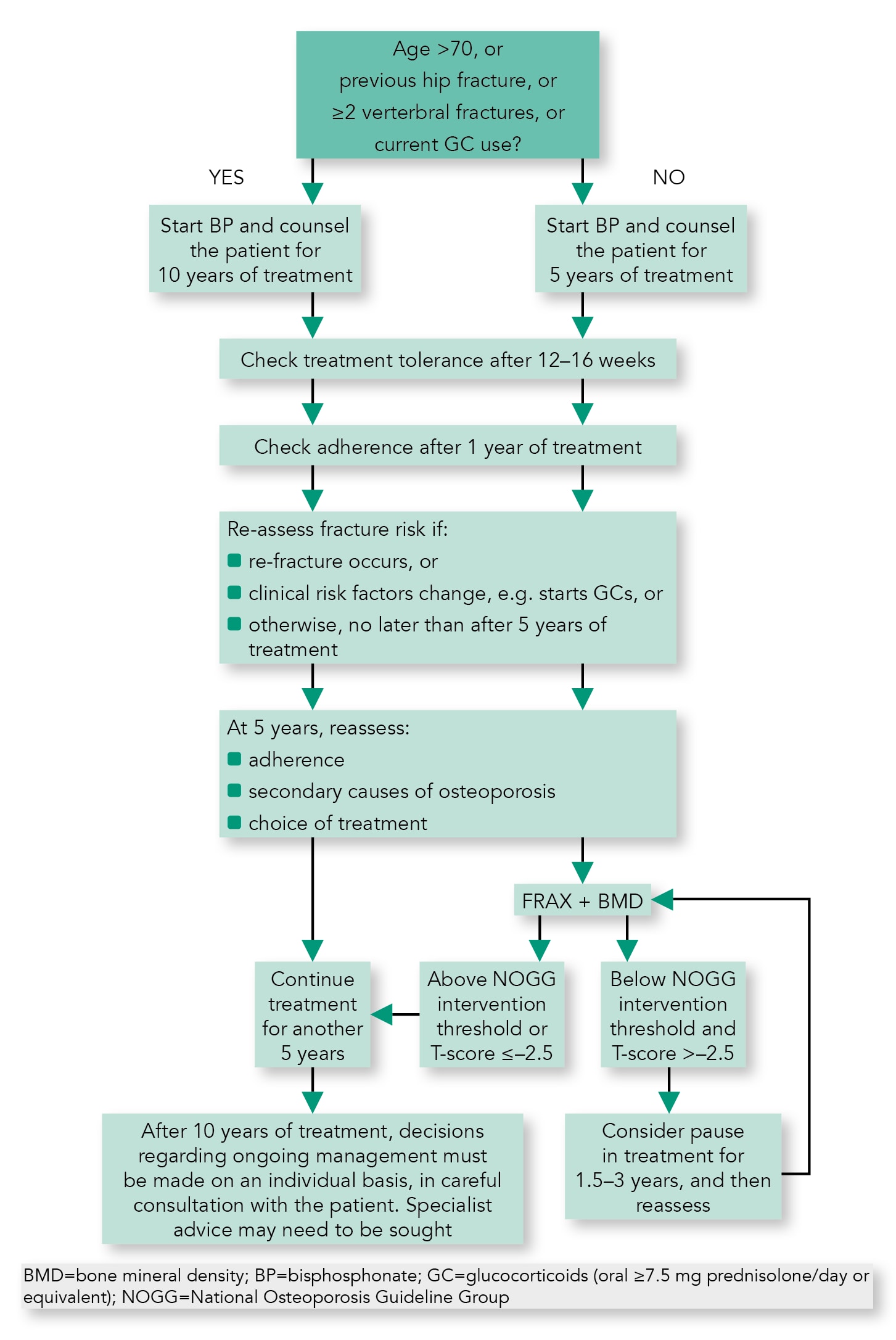
Reassessment of Fracture Risk in Individuals on Osteoporosis Drug Treatment
- Review treatment adherence in men and women who sustain a fragility fracture while on drug treatment (poor adherence is when less than 80% of treatment has been taken correctly), and investigate for secondary causes of osteoporosis [✓]
- Fracture risk assessment in patients receiving drug treatment should be performed using FRAX® with BMD, with arithmetic adjustments to FRAX® probabilities to take account of additional clinical risk factors (see the section, Fracture risk assessment and case finding). If the FRAX®-derived fracture probability exceeds the intervention threshold, drug treatment should be continued [✓]
- If biochemical markers of bone turnover indicate relapse from suppressed bone turnover and/or BMD has decreased following bisphosphonate withdrawal, consider resumption of drug treatment [C]
- After 10 years of bisphosphonate treatment, patient management should be considered on an individual basis [C].
Algorithm 4: Oral Bisphosphonates: Clinical Flowchart for Long-term Treatment and Monitoring
Rare Adverse Effects of Long-term Bisphosphonate and Denosumab Treatment
- During bisphosphonate or denosumab therapy, encourage all patients to maintain good oral hygiene, receive routine dental check-ups, and report any oral symptoms such as dental mobility, pain, or swelling [✓]
- In those with severe dental disease who require bisphosphonate or denosumab treatment, timely dental review and dental treatment by an appropriately experienced dental surgeon should be pursued before drug administration, bearing in mind drug treatment should be initiated as soon as possible after a fragility fracture; a multidisciplinary team approach to discuss individual needs is encouraged [C]
- During bisphosphonate or denosumab treatment, although ideally patients should minimise invasive dental procedures where possible, if indicated they can be carried out safely and successfully in most patients. When dental procedures are required, there are no data available to show whether treatment discontinuation reduces the risk of osteonecrosis of the jaw. Clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment, ensuring patients continue to access routine dental care [C]
- During bisphosphonate or denosumab therapy, advise patients to report any unexplained thigh, groin, or hip pain and if such symptoms develop, the femur should be imaged (by full length femur X-ray, isotope scanning, or magnetic resonance imaging) [✓].
Glucocorticoid-induced Osteoporosis
- Because bone loss and increased fracture risk occur early after initiation of oral glucocorticoids, bone-protective treatment should be started in the following people, at the same time as glucocorticoid therapy without waiting for bone density assessment, which should follow later [✓]:
- anyone with a prior fragility fracture
- women aged 70 years or older
- postmenopausal women, and men aged 50 years or older, prescribed high doses of glucocorticoids, that is, 7.5 mg/day or more of prednisolone or equivalent over 3 months (NB this is equivalent to 30mg/day or more of prednisone for 4 weeks over 3 months)
- postmenopausal women, and men aged 50 years or older, with a FRAX® probability of major osteoporotic fracture or of hip fracture exceeding the intervention threshold
- Oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate are the most cost-effective first-line drug options for bone protection. Denosumab is an alternative option. Teriparatide can be a first-line drug option in those at very high fracture risk [✓]
- Adequate calcium intake should be achieved through dietary intake if possible, with the use of supplements if necessary. An adequate vitamin D status should be maintained, using supplements if required [✓]
- If glucocorticoid therapy is stopped, withdrawal of bone-protective therapy may be considered at the same time, provided that on reassessment of fracture risk using FRAX® the probabilities of both major osteoporotic fracture and of hip fracture lie below the intervention threshold [✓]
- If glucocorticoids are continued long-term, bone protection should be maintained in the majority of cases [✓]
- Patients starting medium- or low-dose oral glucocorticoid therapy who have a FRAX® probability near to, but below, the intervention threshold should have FRAX® with BMD reassessed 12–18 months after starting glucocorticoid therapy [C].
Men Receiving Androgen-deprivation Therapy
NOGG supports the recent guideline published by Brown et al 2020:- All men starting androgen deprivation therapy (ADT) should have their fracture risk assessed using FRAX®, considering ADT use as a secondary cause of osteoporosis, with BMD measured where available [✓]
- Consider referring men with high fracture risk requiring drug treatment to secondary care for assessment and initiation of treatment with bisphosphonates or denosumab [C]
- Men with FRAX® probability near to, but below, the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX® with BMD reassessed 12–18 months after starting ADT [C].
Women Receiving Aromatase Inhibitor Therapy
- All women starting aromatase inhibitor (AI) therapy should have their fracture risk assessed using FRAX®, considering AI use as a secondary cause of osteoporosis, including BMD measurement where practical [✓]
- Women with high fracture risk should be commenced on drug treatment to prevent osteoporosis and fracture, with bisphosphonates or denosumab [✓]
- Women with a FRAX® probability near to, but below, the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX® with BMD reassessed 12–24 months after starting AI therapy [C]
- If adjuvant high-dose bisphosphonate therapy is used as part of breast cancer management, consider assessing fracture risk at the end of this bisphosphonate therapy, particularly if AI therapy continues [C].
For recommendations on specific drug options, management of symptomatic osteoporotic vertebral fractures, and models of care for fracture prevention, refer to the full guideline.

