Latest Guidance UpdatesFebruary 2024: the UK Health Security Agency made minor updates to Testing for pertussis in primary care, and published further Guidance on the management of cases of pertussis in England during the re-emergence of pertussis in 2024 to supplement this guidance; the Guidelines team added sections on recommendations for testing and immunisation of contacts into this summary. |
Overview
This Guidelines summary of UK Health Security (UKHSA)/Public Health England (PHE) guidance on the management of pertussis (whooping cough) covers testing and includes a management algorithm.
In March 2024, the UKHSA produced further guidance to help Health Protection Teams (HPTs) to manage the increase in workload related to pertussis, alongside ongoing raised activity in other vaccine-preventable diseases. The key priority for action during this heightened pertussis activity is to prevent infant hospitalisations and deaths and highlight the importance of timely and complete vaccination in pregnancy, infants, and children aged under 10 years.
Reflecting on Your Learnings
Reflection is important for continuous learning and development, and a critical part of the revalidation process for UK healthcare professionals. Click here to access the Guidelines Reflection Record.
Summary Algorithm for the Management of Cases and Close Contacts of Pertussis
Algorithm 1: Management of Cases and Close Contacts of Pertussis
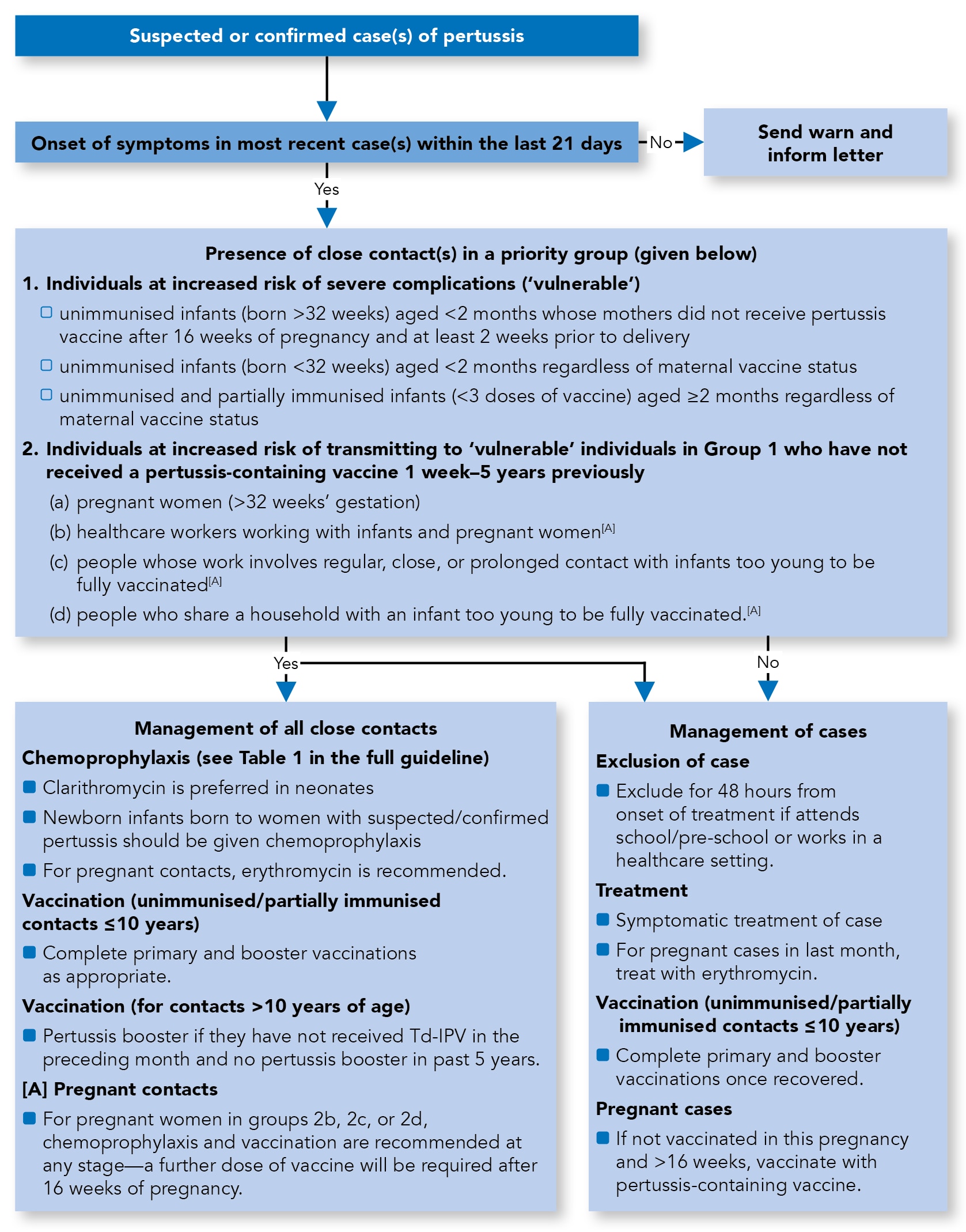
Case Definition
- Suspected case of pertussis:
- any person in whom a clinician suspects pertussis infection or
- any person with an acute cough lasting for 14 days or more, without an apparent cause plus one or more of the following:
- paroxysms of coughing
- post-tussive vomiting
- inspiratory whoop
- AND
- absence of laboratory confirmation
- no epidemiological link to a laboratory-confirmed case
- Confirmed case of pertussis:
- any person with signs and symptoms consistent with pertussis with:
- Bordetella pertussis isolated from a respiratory sample (typically a nasopharyngeal aspirate [NPA] or nasopharyngeal swab/pernasal swab [NPS/PNS; or throat swab]) or
- anti-pertussis toxin Immunoglobulin (Ig) G titre >70 IU/ml from a serum, or >70 aU from an oral fluid (OF) specimen (in the absence of vaccination within the past year)[A] or
- B. pertussis polymerase chain reaction (PCR)-positive in a respiratory clinical specimen
- any person with signs and symptoms consistent with pertussis with:
- Epidemiologically linked case of pertussis:
- a suspected case with signs and symptoms consistent with pertussis, but no laboratory confirmation, who was in contact with a laboratory-confirmed case of pertussis in the 21 days before the onset of symptoms.
Recommended Details to Be Recorded When a Case Is Reported
- Caller details:
- name, address, designation, and contact number
- Demographic details:
- name, date of birth, sex, ethnicity, NHS number
- address, including postcode
- contact details, including phone number
- occupation (if applicable)
- place of work/education (if applicable)
- GP name and contact details (including address and phone number)
- Clinical/epidemiological details:
- clinical information—onset dates, cough (including duration), presence of inspiratory whoop/apnoea/post-tussive vomiting, complications, treatment
- need for admission to hospital (including dates where relevant)
- pertussis immunisation history[B] (including dates)
- pregnancy status
- contact with confirmed or suspected case
- any close contacts within a priority group, including:
- healthcare workers in high-risk settings
- unimmunised infants born after 32 weeks but less than 2 months of age, whose mother did not receive pertussis vaccine after 16 weeks and at least 2 weeks prior to delivery
- unimmunised infants born at 32 weeks or later and less than 2 months of age, regardless of maternal vaccine status
- unimmunised or partially immunised infants aged 2 months and over, regardless of maternal vaccine status
- pregnant women after 32 weeks, and have not received pertussis vaccine at least a week prior to exposure
- context: household, school, healthcare setting (including name).
Risk Assessment for the Index Case
- The positive predictive value (PPV) of a clinical diagnosis of pertussis is not very high, particularly among adolescents and adults who may present with atypical features. However, the PPV will increase during periods of heightened pertussis activity and will vary with age
- Risk assessment should be based on a combination of clinical and epidemiological factors such as clinical presentation, vaccination history, and epidemiological links. Management of the index case and any vulnerable contacts should proceed based on this risk assessment without waiting for the results of laboratory testing, and prompt public health actions to prevent onward transmission should be considered.
Laboratory Confirmation and Public Health Action
- Appropriate public health action should not wait for laboratory results, as negative results cannot be used to exclude pertussis infection. In the event of an outbreak, the local HPT and the testing laboratory should be informed in order that testing can be appropriately prioritised
- Please contact the Respiratory and Vaccine Preventable Bacteria Reference Unit (RVPBRU) on 0208 327 7327 and discuss with senior staff prior to sending serological specimens for priority testing
- please note, these services are not available outside of regular working hours at PHE Colindale
- see the user manual for details.
Recommendations for Testing
Infants and Children Aged Under 2 Years
- PCR testing is recommended for infants and children with suspected pertussis in the early stages of the illness and less than 21 days post-cough onset
- If local laboratory facilities permit, culture should also be performed. Please ask local laboratory for any putative B. pertussis isolates (pure cultures) to be sent to RVPBRU for confirmation
- In those who present late, serology can be undertaken (more than 14 days post-cough onset) but is not usually recommended for infants under 12 months as the antibody response of infants may not be typical of that seen in older children and adults. Serology is not recommended in children who have received a pertussis-containing vaccine in the previous year, as the results may be confounded by recent vaccination and therefore are unlikely to be useful in children under the age of 2 years. Liaise with local NHS or regional PHE microbiologist, HPT staff, or RVPBRU for further advice.
Children Aged 2 Years or More and Adults
- PCR is recommended in the early stages of illness (less than 21 days post-cough onset) and within 48 hours of antibiotic therapy
- If local laboratory facilities permit, culture should also be performed. Please ask local laboratory for any putative B. pertussis isolates (pure cultures) to be sent to RVPBRU for confirmation
- For children aged 2 to less than 17 years, OF or serology is recommended for notified cases where the onset of cough is greater than 14 days AND who have not been immunised against pertussis in the previous year
- For children aged 17 years or older and adults, serology is recommended where the onset of cough is greater than 14 days AND who have not been immunised against pertussis in the previous year.
Swab Types and Sampling for Culture and PCR
- The posterior nasopharynx should be sampled using an NPS/PNS
- The Copan-style swab is also acceptable, or an NPA.
Case Management
Exclusion
See Algorithm 1 and the full guideline for further details.Antibiotic Therapy
- The decision to offer antibiotics and the choice of treatment is a clinical decision
- Ideally, antibiotics should be administered as soon as possible after onset of illness in order to eradicate the organism and limit ongoing transmission
- The effect of treatment on reducing symptoms, however, is limited or lacking, especially when given late during the disease
- For suspected, epidemiologically linked, or confirmed cases, recommended antibiotic regimens are summarised in Table 1
- Antibiotics are not recommended or thought to be beneficial after 3 weeks of symptoms.
Immunisation
- It is important that unvaccinated and partially immunised cases up to 10 years of age complete their course of primary immunisation and booster vaccine once they have recovered from their acute illness, following the PHE guidance document Vaccination of individuals with uncertain or incomplete immunisation status
- Pregnant women who have been diagnosed with pertussis (at any stage of pregnancy) and have not been vaccinated after 16 weeks of pregnancy should be offered a dose of pertussis-containing vaccine in line with national recommendations
- Pregnant women diagnosed with pertussis before 16 weeks' gestation should wait until they reach 16 weeks of pregnancy (and ideally following the detailed ultrasound scan) to have the vaccine.
Table 1: Recommended Antibiotic Treatment and PEP by Age Group[A]
| Age Group | Clarithromycin[B] | Azithromycin[B] | Erythromycin | Co-trimoxazole[B],[D] |
|---|---|---|---|---|
| Neonates (<1 month) | Preferred in neonates 7.5 mg/kg b.d. for 7 days | 10 mg/kg o.d. for 3 days | Not recommended due to association with hypertrophic pyloric stenosis | Not licensed for infants below 6 weeks |
| Infants (1–12 months) and children (>12 months) | 1 month–11 years:
| 1–6 months: 10 mg/kg o.d. for 3 days >6 months: 10 mg/kg (max 500 mg) o.d. for 3 days | 1–23 months: 125 mg every 6 hours for 7 days[C] 2–7 years: 250 mg every 6 hours for 7 days[C] 8–17 years: 500 mg every 6 hours for 7 days[C] | 6 weeks–5 months: 120 mg b.d. for 7 days 6 months–5 years: 240 mg b.d. for 7 days 6–11 years: 480 mg b.d. for 7 days 12–17 years: 960 mg b.d. for 7 days |
| Adults | 500 mg b.d. for 7 days | 500 mg o.d. for 3 days | 500 mg every 6 hours for 7 days[C] | 960 mg b.d. for 7 days |
| Pregnant women[E] | Not recommended | Not recommended | Preferred antibiotic—not known to be harmful | Contraindicated in pregnancy |
| [A] The above information has been taken from BNF 75 (March 2018) and BNF for Children 2017–2018. The recommendation to use azithromycin for infants <6 months of age is based on advice from experts on the Pertussis Guidelines Group and CDC guidelines. Azithromycin and co-trimoxazole doses are extrapolated from treatment of respiratory tract infections [B] Please note that the doses for treatment and prophylaxis are the same [C] Doses can be doubled in severe infections [D] Consider if macrolides contraindicated or not tolerated b.d.=twice a day; BNF=British National Formulary; CDC=Centers for Disease Control and Prevention; o.d.=once a day; PEP=post-exposure prophylaxis | ||||
Contact Management
- Management of contacts should proceed for all clinically suspected, epidemiologically linked, and laboratory-confirmed cases.
Definition of Close Contacts
- Family members or people living in the same household are considered close ‘household contacts’
- Contacts in institutional settings with an overnight stay in the same room, such as boarding school dormitories, during the infectious period should also be considered close contacts
- Other types of contact, such as contact at work or school, would generally not be considered close contact, although each situation would need to be assessed on an individual basis where vulnerable contacts are involved
- For the definition of a significant exposure in a healthcare setting, please refer to PHE Guidelines for the public health management of pertussis incidents in healthcare settings.
Definition of Contacts Considered as Priority Groups for Public Health Action
- These include individuals who are themselves at increased risk of complications following pertussis (Group 1) as well as those at risk of transmitting the infection to others at risk of severe disease (Group 2)
- Contacts of parapertussis do not require public health action.
Group 1
- Individuals at increased risk of severe complications (‘vulnerable’):
- unimmunised infants (born after 32 weeks) less than 2 months of age whose mothers did not receive pertussis vaccine after 16 weeks of pregnancy and at least 2 weeks prior to delivery
- unimmunised infants (born before 32 weeks) less than 2 months of age regardless of maternal vaccine status
- unimmunised and partially immunised infants (less than three doses of vaccine) aged 2 months and above regardless of maternal vaccine status.
Group 2
- Individuals at increased risk of transmitting to ‘vulnerable’ individuals in Group 1 who have not received a pertussis-containing vaccine more than 1 week and less than 5 years ago:
- pregnant women (over 32 weeks' gestation)
- healthcare workers working with infants and pregnant women
- people whose work involves regular, close, or prolonged contact with infants too young to be fully vaccinated
- people who share a household with an infant too young to be fully vaccinated.
Exclusion of Contacts
- Exclusion for asymptomatic contacts is NOT required.
Chemoprophylaxis of Contacts
- Given the limited benefit of chemoprophylaxis, antibiotic prophylaxis should only be offered to close contacts when both of the following conditions apply:
- onset of disease in the index case is within the preceding 21 days AND
- there is a close contact in one of the priority groups as defined above
- Where both these conditions are met, ALL close contacts of a confirmed case (regardless of age and previous immunisation history) should be offered chemoprophylaxis. The dose of antibiotics for use as chemoprophylaxis is the same as for the treatment of cases (see Table 1). Chemoprophylaxis is NOT required where there are no close contacts in the priority groups defined above, or for healthy contacts.
Immunisation of Contacts
- Immunisation should be considered for those who have been offered chemoprophylaxis:
- unimmunised and partially immunised contacts aged 10 years or less should complete the schedule with the appropriate vaccine
- a booster dose of pertussis-containing vaccine is recommended for individuals aged 10 years or over who have not received a dose of pertussis-containing vaccine in the last 5 years and no Td-IPV vaccine in the preceding month.
Special Situations
Please see full guideline for further details on special situations (including healthcare, nursery, and school settings).Outbreaks
- Where disease transmission is widespread, the benefit of wider chemoprophylaxis is likely to be of limited value
- In the event of a hospital or community outbreak, an outbreak control team should be convened at the earliest opportunity and the local HPT informed
- The priority in these circumstances is active case finding and therefore a less specific case definition should be used to ensure no cases are missed
- Once laboratory confirmation of pertussis infection has been demonstrated in a cluster (such as a school), it is not usually necessary to perform extensive additional testing
- Expert advice on outbreak investigation and management is available from Immunisation Services, NIS Colindale, PHE (020 8200 6868/4400) and on laboratory investigation from the RVPBRU (0208 327 7327).
Testing for Pertussis in Primary Care
- Suspect pertussis in patients with a cough illness lasting 14 days or more without an apparent cause plus one of the following:
- paroxysms of coughing
- inspiratory ‘whoop’
- post-tussive vomiting
- ALL CASES should be notified to your local HPT
- When notifying, it is helpful to let the HPT know if the case has had contact with pregnant individuals or children aged under 1 year, including through occupational exposure (for example, healthcare or nursery settings)
- Recommended tests for pertussis testing vary according to the length of time since cough onset:
- less than 2 weeks from cough onset—PCR and culture
- between 2 and 3 weeks from cough onset—PCR and culture and either OF kit (OFK; if aged 2 to under 17 years) or serology
- more than 3 weeks from cough onset—either OFK (if aged 2 to under 17 years of age) or serology.
Sending a Pertussis PCR Test—Free Service
- Please submit samples to your local laboratory as per normal protocol
- Samples will then be referred for pertussis PCR detection by your local public health laboratory (PHL)
- Pertussis PCR testing is not chargeable when performed at a PHL
- Please label clearly ‘for Bordetella pertussis PCR testing’
- PCR testing can be performed on the following specimens:
- throat swabs
- collected using a virology swab or dry swab in a sterile container
- pernasal swab
- use a dry swab with a flexible wire shaft and a rayon, dacron, or nylon bud
- a rigid shaft is not suitable
- push the swab along the floor of the nasal cavity, as far towards the posterior wall of the nasopharynx as possible
- nasopharyngeal swab
- use a dry or Copan-style NPS
- see this Centers for Disease Control and Prevention (CDC) video for further guidance
- nasopharyngeal aspirate
- provide not less than 400 mcl in a sterile container
- see this CDC video for further guidance.
- throat swabs
Sending a Pertussis Culture
- An NPS or PNS may be taken for culture
- The swab should be placed in a culture medium (ideally charcoal) and submitted to your local microbiology lab
- Please clearly label as ‘for pertussis culture’.
Requesting an Oral Fluid Kit—Free Service
- For cases aged 2 years to less than 17 years, notify the case to your local HPT and they will post an OFK directly to the case
- Note that OF testing is not recommended if the case has been immunised against pertussis in the previous year, as a positive result cannot be interpreted.
Sending a Pertussis Serology Test
- For cases not aged 2 years to less than 17 years, a charged-for serology test using serum can be arranged via your local laboratory, either undertaken by them or then sent on to the RVPBRU. Form R3 can be used
- Note that serology is not recommended if the case has been immunised against pertussis in the previous year, as a positive result cannot be interpreted.
Managing Cases
- If 3 weeks or less from symptom onset, treat with appropriate antibiotics once PCR and culture tests have been taken. Exclude the case from school or work until they have completed 2 days of the antibiotic course. Work with the local HPT to identify and manage vulnerable close contacts. There is no need to prescribe a second course of antibiotics even if symptoms are not resolving
- If more than 3 weeks from symptom onset, antibiotics are not required to manage pertussis even if the case still has symptoms. No exclusion of the case is necessary.
Further information is available in the Pertussis guidelines for public health management on the testing for and management of pertussis; or, please call your local HPT for further advice.
Footnotes
[A] This is currently under review and will be modified as more data are available
[B] Including pertussis vaccines administered to a mother during pregnancy for cases born after 30 September 2012.

