Overview
The following summary of the Primary Care Women's Health Forum's Menopause—guidance on management and prescribing HRT for GPs is based on NICE Guideline 23, Menopause: diagnosis and management. It includes information on diagnosis, oestrogens and progestogens used in hormone replacement therapy (HRT), choosing the appropriate treatment, and possible medications to use.
For a complete set of recommendations, refer to the full guideline.
Diagnosis
- Diagnosis can be made without laboratory tests in otherwise healthy women aged over 45 years with appropriate symptoms:
- perimenopause based on vasomotor symptoms and irregular periods
- menopause in women who have:
- not had a period for at least 12 months and are not using hormonal contraception or
- symptoms in women without a uterus
- Consider using a follicle-stimulating hormone (FSH) test to diagnose menopause only:
- in women aged 40–45 years with possible menopausal symptoms, including a change in their menstrual cycle
- in women aged under 40 years in whom menopause is suspected
- If possible, test day 2–5 of the cycle
- Two FSH levels over 30 mIU/ml taken 6 weeks apart in women with amenorrhoea using hormonal contraception can be used to determine when contraception can be stopped (after a further 1 year in women over 50 years, 2 years in those under 50 years)
- Some women may have normal levels of FSH during the menopausal transition, so this should not exclude the perimenopause as a cause of their symptoms.
Vasomotor Symptoms
- Offer women HRT after discussing with them the short-term (up to 5 years) and longer-term benefits and risks
- Offer a choice of preparations as follows:
- oestrogen and progestogen to women with a uterus
- oestrogen alone to women without a uterus
- Do not offer selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, or clonidine as first-line treatment for vasomotor symptoms
- Consider testosterone supplementation for menopausal women with low sexual desire if HRT alone is not sufficient.
Genitourinary Syndrome of Menopause
- Vaginal oestrogen can be given to women with urogenital atrophy (including those on systemic HRT) and continued for as long as needed to relieve symptoms. Treatment should be started early before irreversible changes have occurred
- If vaginal oestrogen does not relieve symptoms of urogenital atrophy, consider increasing the dose beyond the licensed twice-weekly dose after seeking advice from a healthcare professional with expertise in menopause. Vaginal moisturisers and lubricants can be used alone or in addition to vaginal oestrogen
- There is no need for monitoring of endometrial thickness during treatment of urogenital atrophy with vaginal oestrogens. However, advise women they should report unscheduled vaginal bleeding promptly.
Venous Thromboembolism
- The risk of venous thromboembolism (VTE) is increased by oral HRT compared with baseline population risk (relative risk=2)
- The risk of VTE associated with HRT is more significant for oral than transdermal preparations
- The risk associated with transdermal HRT given at standard therapeutic doses (under 50 mcg) is no greater than the baseline population risk
- Consider transdermal rather than oral HRT for menopausal women who are at increased risk of VTE, including those with a body mass index over 30 kg/m2
- Consider referring menopausal women at high risk of VTE (for example, those with a strong family history of VTE or a hereditary thrombophilia) to a haematologist for assessment before considering HRT.
Cardiovascular Disease
- HRT does not increase coronary heart disease risk when started in women aged under 60 years, and does not affect the risk of dying from cardiovascular disease
- The presence of cardiovascular risk factors is not a contraindication to HRT as long as they are optimally managed
- The baseline risk of coronary heart disease and stroke for women around menopausal age varies according to their personal cardiovascular risk factors
- HRT with oestrogen alone is associated with no, or reduced, risk of coronary heart disease
- HRT with oestrogen and progestogen is associated with little or no increase in the risk of coronary heart disease
- Taking oral oestrogen (but not transdermal under 50 mcg/24 hours) is associated with a small increase in the risk of stroke, but the baseline population risk of stroke in women aged under 60 years is very low, so the increased risk is nonsignificant.
Type 2 Diabetes
- Taking HRT (either orally or transdermally) is not associated with an increased risk of developing type 2 diabetes
- In women with diabetes, HRT is not associated with an adverse effect on blood glucose control.
Osteoporosis
- Give women advice on bone health and discuss any risk factors for osteoporosis
- The baseline population risk of fragility fracture for women around menopausal age in the UK is low and varies according to their personal and familial risk factors
- Risk of fragility fracture is decreased while taking HRT but increases once treatment stops, although benefits may persist for a while in women who take HRT for longer
- HRT is the only treatment that gives protection against all fractures, even in women with normal bone density
- Women who have undergone an early menopause, either natural or surgical, should be prescribed HRT and advised to continue until at least the normal age of the menopause. Women with established osteoporosis aged under 60 years, especially with menopausal symptoms, should be given HRT to improve their bone density.
Loss of Muscle Mass and Strength
- There is limited evidence suggesting that HRT may maintain muscle mass and strength, which otherwise tends to decrease after the menopause. Muscle mass and strength is also maintained through daily activities and weight-bearing exercise.
Breast Cancer
- The baseline risk of breast cancer for women around menopausal age varies according to the presence of underlying familial and environmental risk factors
- There is still no evidence of any increase in mortality from breast cancer in women taking HRT. NICE stated that:
- HRT with oestrogen alone is associated with little or no increase in the risk of breast cancer
- HRT with oestrogen and progestogen can be associated with an increase in the risk of breast cancer; however, any increase in risk of breast cancer is related to treatment duration and reduces after stopping HRT.
Ovarian Cancer
- A 2015 meta-analysis of 52 epidemiological studies has shown an increased risk of ovarian cancer with oestrogen-only and combined HRT. While this study provides evidence of an association between HRT use and some tumour subtypes, it provides insufficient evidence to claim that HRT causes ovarian cancer
- When counselling patients, it is essential to discuss these findings in terms of absolute risk
- With 5 years of HRT use, there could be one additional ovarian cancer per 1000 users and one additional death per 1700 users among women of all ages.
Premature Ovarian Insufficiency
- In women with premature ovarian insufficiency (menopause under 40 years) it is essential to start hormonal treatment either with HRT or a combined hormonal contraceptive
- This treatment should be continued until at least the age of natural menopause (unless contraindicated) to protect against the increased risk of dementia, cognitive decline, cardiovascular disease, and osteoporosis seen in these women
- HRT has a negligible effect on blood pressure and beneficial effects on metabolic parameters, when compared with a combined oral contraceptive
- Both HRT and combined oral contraceptives offer bone protection
- HRT is not a contraceptive
- Consider referring women with premature ovarian insufficiency to healthcare professionals who have the relevant experience to help them manage all aspects of physical and psychosocial health related to their condition.
| Review Each Treatment for Menopausal Symptoms |
|---|
|
Oestrogens and Progestogens
All routes of oestrogen administration are equally effective for symptom relief and bone protection, but their metabolic effects differ.- Oral oestrogen increases sex hormone binding globulin levels, which can result in lower free testosterone concentrations—a benefit if women are noticing postmenopausal hirsutism
- Transdermal preparations can be started at a low dose, such as oestradiol 25 mcg patch, or one measure of oestradiol gel and titrated up until symptoms are alleviated. If symptoms are still present after 1 month, an increase to 37.5 mcg, then 50 mcg (patch or gel) is associated with fewer side effects such as breast tenderness and vaginal bleeding, and also keeps risks minimised
- The increased VTE risk seen with oral preparations is not found using transdermal preparations under 50 mcg oestradiol gel
- Most women find a standard dose of a 50 mcg patch or two measures of oestradiol gel are adequate for symptom relief; the only exception is younger women either after oophorectomy or with premature ovarian insufficiency who often require higher doses of oestradiol
- In women who are still experiencing some periods, a cyclical progestogen regime is required in order to regulate the cycles, encouraging a bleed around the end of each month. Cyclical oral formulations contain older progestogens, which protect the endometrium but may have progestogenic side effects
- For perimenopausal women, micronised progesterone 200 mg a day can be used for 14 days of each month. In women who are more than a year post-last period or have used a cyclical preparation for 2–3 years this can be transferred to a continuous regime using micronised progesterone 100 mg daily, which should keep women amenorrhoeic
- Micronised progesterone or dydrogesterone should be the first choices of progestogen as they appear to be safer for the cardiovascular system, having neutral effect on lipids and coagulation, and a lower risk of breast cancer.
Table 1: Oestrogens and Progestogens
| Type of Progestogen | Good For | Watch Out For | Prescribed As |
|---|---|---|---|
| Synthetic: C19 Testosterone Derivatives | |||
| Norethisterone | Cycle control Androgenic—good for libido | Some degree of oestrogenic effect caution if VTE risk ++ | E2/net fixed combined tablet/patch or as standalone |
| LevonorgestrelAs IUS | Cycle control Low systemic absorption Excellent contraception Good for HMB | Androgenic effects (acne, mood) PMS | 52 mg LNG IUS Or In combined patch |
| Synthetic: C21 Progesterone Derivatives | |||
| Medroxyprogesterone acetate | Cycle control | Caution if VTE risk + | SCHRT—E2 plus 10–20 mg cyclically CCHRT–E2 plus 5 mg conti Or fixed dose in oral |
| Dydrogesterone | Non-androgenic so good if PMS-type side effects | Only available with oral oestrogen | Oral fixed dose Sequential (including low dose) or combined |
| Natural progesterone: Micronised progesterone | Fewer progestogenic side effects No androgenic or glucocorticoid activity No impact on lipids | Less effective cycle control Take at night as may cause sedation | Sequential—200 mg cyclically Or Continuous 100 mg daily |
| CCHRT=continuous combined hormone replacement therapy; HMB=heavy menstrual bleeding; IUS=intrauterine system; LNG IUS=levonorgestrel-releasing intrauterine system; PMS=premenstrual syndrome; SCHRT=sildenafil citrate hormone replacement therapy; VTE=venous thromboembolism | |||
Table 2: Progestogens Currently Licensed For Use in the UK as part of Combined Hormone Replacement Therapy
| Progestogen | Oestrogenic | Anti-oestrogenic | Androgenic | Anti-androgenic | Glucocorticoid | Anti-mineralocorticoid |
|---|---|---|---|---|---|---|
| Norethisterone | ✓ | ✓ | ✓ | x | x | x |
| Levonorgestrel/ norgestrel (including intrauterine devices) | x | ✓ | ✓ | x | x | x |
| Progesterone | x | ✓ | x | ✓ | ✓ | ✓ |
| Medroxyprogesterone acetate | x | ✓ | ✓ | x | ✓ | x |
| Dydrogesterone | x | ✓ | x | ✓ | x | ✓ |
Algorithm 1: Flowchart for Hormone Replacement Therapy Prescribing
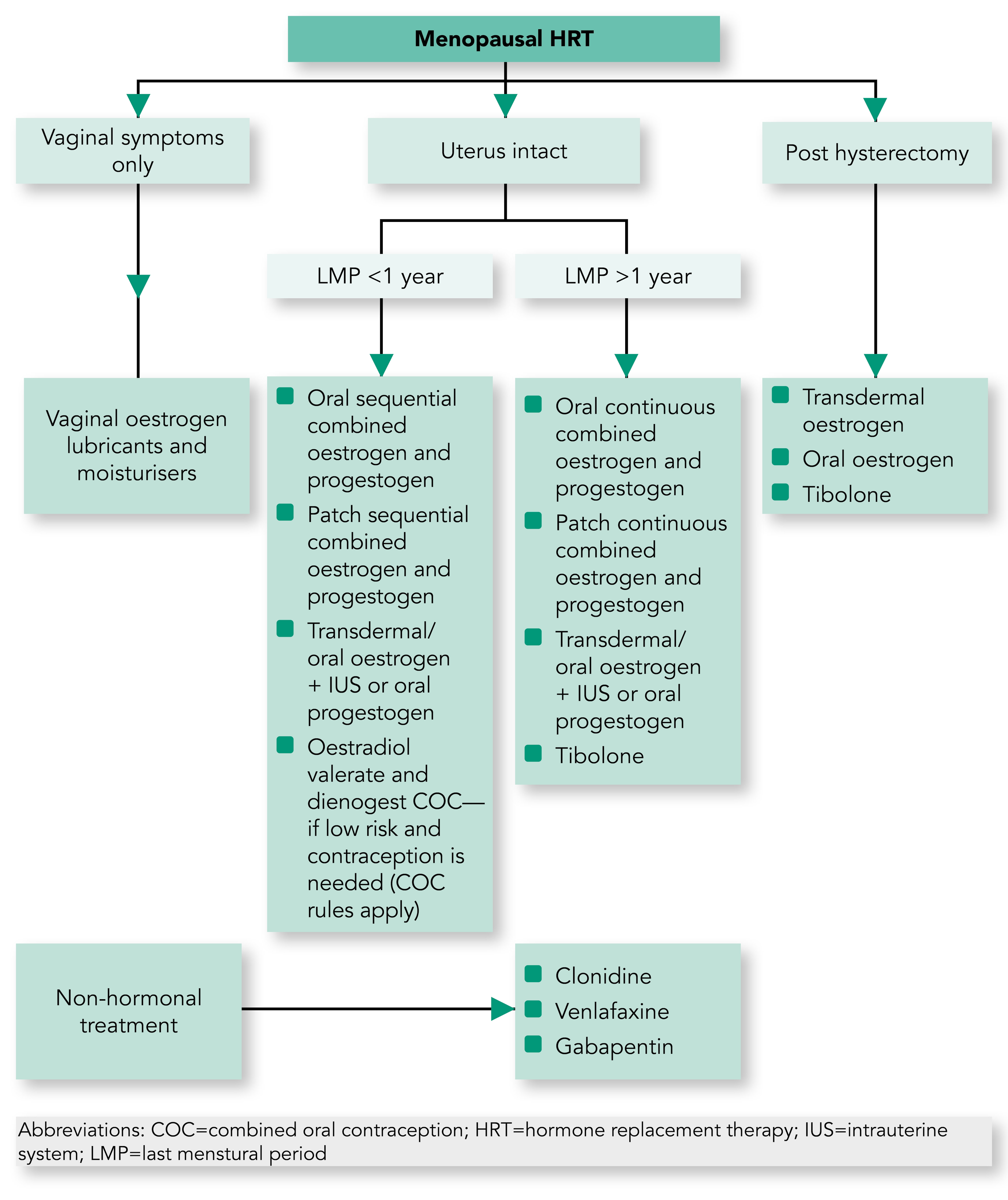
Table 3: Possible Medications to Use
| HRT | Tablets[A] | Second Line[A] | Transdermal[A] |
|---|---|---|---|
| SEQUENTIAL PREPARATIONS For patients with:
| Oestradiol 1 mg + norethisterone sequential Oestradiol 2 mg + norethisterone sequential | Oestradiol + dydrogesterone 1/10 mg sequential Oestradiol + dydrogesterone 2/10 mg sequential (non-androgenic) | Oestradiol 50 mcg + levonorgestrel 10 mcg sequi patch Oestradiol 50 mcg + norethisterone 170 mcg sequi patch |
CONTINUOUS COMBINEDBleed free HRT use if:
| Oestradiol 2 mg + norethisterone 1 mg conti Oestradiol 0.5 mg + dydrogesterone 2.5 mg conti Oestradiol 1 mg + dydrogesterone 5 mg conti | Tibolone 2.5 mg Can be useful if:
| Oestradiol 50mcg + levonorgestrel 7 mcg conti patch Oestradiol 50 mcg + norethisterone 170 mcg conti patch |
| UNOPPOSED OESTROGEN Post hysterectomy | Oestradiol 1 mg tablets Oestradiol 2 mg tablets | Oestradiol patch 25 mcg, 37.5 mcg, 50 mcg, 75 mcg, 100 mcg Oestradiol 0.06% gel Oestradiol 500 mcg or 1 mg gel sachets | |
| TOPICAL VAGINAL OESTROGEN | Oestradiol 10 mcg vaginal pessaries Oestriol 0.1% cream Oestradiol vaginal ring | ||
| PROGESTOGEN ADJUNCT TO TOPICAL OESTROGEN IF NO HYSTERECTOMY | Medroxyprogesterone 10 mg day 14–28 or 2.5–5 mg daily[B] Micronised progesterone 200 mg day 14–28 or 100 mg daily[B] | Levonorgestrel intrauterine system 52 mg Replace after 5 years as per FSRH guidance | |
| [A] Use the lowest dose to control symptoms [B] Day 14–28 (sequential—i.e. still giving periods), daily for continuous combined (bleed-free) | |||
| FSRH=Faculty of Sexual and Reproductive Health; HRT=hormone replacement therapy | |||

