Overview
This Guidelines summary covers key recommendations for primary care on the management of anaemia in people with chronic kidney disease (CKD).
Key recommendations include: diagnosing and assessing anaemia; managing anaemia; monitoring anaemia treatment; hyperphosphataemia in people with CKD stage 4 or 5; and other complications in adults.
View the related Guidelines summary, Chronic Kidney Disease: Assessment and Management, for recommendations on: investigations for chronic kidney disease; classification of CKD in adults; frequency of monitoring; information and education for people with CKD; risk assessment, referral criteria and shared care; and pharmacotherapy.
For a complete set of recommendations, refer to the full guideline.
This guideline updates and replaces NICE Clinical Guideline 182 and NICE Guideline 8.
Diagnosing and Assessing Anaemia
Diagnostic Role of Haemoglobin Levels
- Consider investigating and managing anaemia in adults, children and young people with CKD if:
- their haemoglobin (Hb) level falls to 110 g/litre or less (or 105 g/litre or less if younger than 2 years) or
- they develop symptoms attributable to anaemia (such as tiredness, shortness of breath, lethargy and palpitations).
Diagnostic Role of Glomerular Filtration Rate
- In adults, children and young people with anaemia (see the first recommendation in the below section, Diagnotic tests to determine iron status and predict response to iron therapy):
- If eGFR is above 60 ml/min/1.73 m2, investigate other causes of anaemia as it is unlikely to be caused by CKD.
- If eGFR is between 30 and 60 ml/min/1.73 m2:
- investigate other causes of anaemia, but
- use clinical judgement to decide how extensive this investigation should be, because the anaemia may be caused by CKD.
- If eGFR is below 30 ml/min/1.73 m2, think about other causes of anaemia but note that anaemia is often caused by CKD.
Diagnostic Tests to Determine Iron Status and Predict Response to Iron Therapy
- Carry out testing to diagnose iron deficiency and determine potential responsiveness to iron therapy and long-term iron requirements every 3 months (every 1 to 3 months for people having haemodialysis).
- Use percentage of hypochromic red blood cells (% HRC; more than 6%), but only if processing of blood sample is possible within 6 hours.
- If using percentage of hypochromic red blood cells is not possible, use reticulocyte Hb content (CHr; less than 29 pg) or equivalent tests – for example, reticulocyte Hb equivalent.
- If these tests are not available or the person has thalassaemia or thalassaemia trait, use a combination of transferrin saturation (less than 20%) and serum ferritin measurement (less than 100 micrograms/litre).
- Do not request transferrin saturation or serum ferritin measurement alone to assess iron deficiency status in people with anaemia of CKD.
- Do not routinely measure erythropoietin levels for the diagnosis or management of anaemia in people with anaemia of CKD.
Managing Anaemia
Starting Erythropoietic Stimulating Agent Therapy in Iron Deficiency
- ESA (erythropoietic stimulating agent) therapy should not be started in the presence of absolute iron deficiency without also managing the iron deficiency.
Maximum Iron Levels in People With Anaemia of CKD
- In adults, children and young people treated with iron, serum ferritin levels should not rise above 800 micrograms/litre. In order to prevent this, review the dose of iron when serum ferritin levels reach 500 micrograms/litre.
Clinical Utility of ESA Therapy in People With Sufficient Iron
- Discuss the pros and cons of a trial of anaemia management with the person with anaemia of CKD, and their families and carers if agreed.
- ESAs need not be administered if the presence of comorbidities, or the prognosis, is likely to negate the benefits of correcting the anaemia.
- Start a trial of anaemia correction when there is uncertainty over whether the presence of comorbidities, or the prognosis, would negate benefit from correcting the anaemia with ESAs.
- If a trial of ESA therapy is carried out, assess the effectiveness of the trial after an agreed interval. Agree with the person with anaemia of CKD (and their families and carers, if appropriate) whether or not to continue ESA therapy.
- Review treatment in all people started on ESA therapy after an agreed interval to decide whether or not to continue using ESAs.
Nutritional Supplements
- Do not prescribe supplements of vitamin C, folic acid or carnitine as adjuvants specifically for the treatment of anaemia of CKD.
Androgens
- Do not use androgens to treat anaemia in people with anaemia of CKD.
Hyperparathyroidism
- Treat clinically relevant hyperparathyroidism in adults, children and young people with CKD to improve the management of the anaemia.
Person-centred Care and ESAs
- Give adults, children and young people offered ESA therapy and their GPs information about why ESA therapy is needed, how it works and what benefit and side effects may be experienced.
- When managing the treatment of anaemia of CKD, there should be agreed protocols defining roles and responsibilities of healthcare professionals in primary and secondary care.
- Explain to people receiving ESA therapy about the importance of concordance with therapy and the consequences of poor adherence.
- When prescribing ESA therapy, take into account the person’s preferences about supervised- or self-administration, dose frequency, pain on injection, method of supplying ESA and storage. In order for people to self-administer their ESA in a way that is clinically effective and safe, make arrangements to provide ready, reasonable and uninterrupted access to supplies.
Patient Education Programmes
- Offer culturally and age-appropriate patient education programmes to all adults, children and young people diagnosed with anaemia of CKD (and their families and carers). These should be repeated as requested, and according to the person’s changing circumstances. They should include the following key areas:
- Practical information about how anaemia of CKD is managed.
- Knowledge (for example, about symptoms, iron management, causes of anaemia, associated medications, phases of treatment).
- Professional support (for example, contact information, community services, continuity of care, monitoring, feedback on progress of results).
- Lifestyle (for example, diet, physical exercise, maintaining normality, meeting other people with the condition).
- Adaptation to chronic disease (for example, previous information and expectations, resolution of symptoms).
Monitoring Anaemia Treatment
Monitoring Iron Status
- Do not check iron levels earlier than 1 week after administering intravenous iron in adults, children and young people with anaemia of CKD. The length of time to monitoring of iron status is dependent on the product used and the amount of iron given.
- Carry out routine monitoring of iron stores to prevent iron overload using serum ferritin at intervals of 1 to 3 months.
Monitoring Hb Levels
- In adults, children and young people with anaemia of CKD, monitor Hb:
- every 2 to 4 weeks in the induction phase of ESA therapy
- every 1 to 3 months in the maintenance phase of ESA therapy
- more frequently after an ESA dose adjustment
- in a clinical setting chosen in discussion with the person, taking into account their convenience and local healthcare systems.
Detecting ESA Resistance
- After other causes of anaemia, such as intercurrent illness or chronic blood loss have been excluded, regard people with anaemia of CKD as resistant to ESAs when:
- an aspirational Hb range is not achieved despite treatment with 300 IU/kg/week or more of subcutaneous epoetin or 450 IU/kg/week or more of intravenous epoetin or 1.5 micrograms/kg/week of darbepoetin or
- there is a continued need for the administration of high doses of ESAs to maintain the aspirational Hb range.
- In people with CKD, pure red cell aplasia (PRCA) is indicated by a low reticulocyte count, together with anaemia and the presence of neutralising antibodies. Confirm PRCA by the presence of anti-erythropoietin antibodies together with a lack of pro-erythroid progenitor cells in the bone marrow.
- In people with anaemia of CKD, aluminium toxicity should be considered as a potential cause of a reduced response to ESAs after other causes, such as intercurrent illness and chronic blood loss, have been excluded.
Managing ESA Resistance
- If aluminium toxicity is suspected in an adult, child or young person with anaemia of CKD having haemodialysis, perform a desferrioxamine test and review the management of their condition accordingly.
- Consider specialist referral for people with ESA-induced PRCA.
Role of Blood Transfusion in Managing ESA Resistance
- Consider referring adults, children and young people with ESA resistance to a haematology service, particularly if an underlying haematological disorder is suspected.
- Evaluate and discuss the risks and benefits of red cell transfusion with the person or, if appropriate, with their family or carers.
- Take into account the person’s symptoms, quality of life, underlying conditions and the chance of a future successful kidney transplant, in addition to Hb levels, when thinking about the need for red cell transfusion.
Hyperphosphataemia in People With CKD Stage 4 or 5
Dietary Management for Adults, Children and Young People
- A specialist renal dietitian, supported by healthcare professionals with the necessary skills and competencies, should carry out a dietary assessment and give individualised information and advice on dietary phosphate management.
Algorithm 1: Phosphate Binders Visual Summary
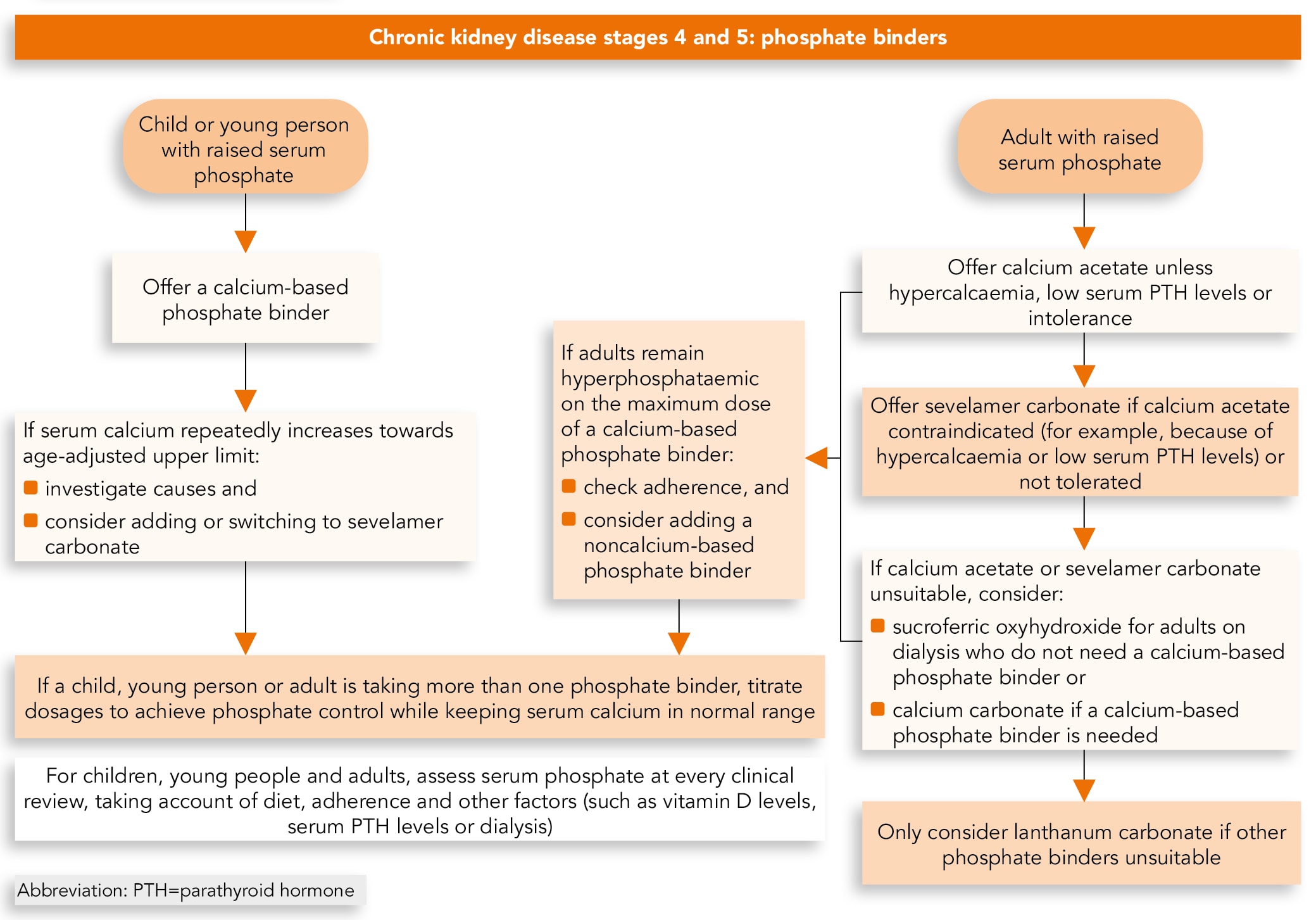
Before Starting Phosphate Binders for Adults, Children and Young People
- Before starting phosphate binders for adults, children and young people with CKD stage 4 or 5, optimise:
- diet (see recommendations 1.4.7–1.4.9 in the full guideline for adults)
- dialysis, for people who are having this.
- When offering a phosphate binder, explain to them and their family members or carers (as appropriate):
- the reason for offering phosphate binders
- the risks if they are not taken
- the side effects linked to phosphate binders
- when and how they have to be taken (depending on the type of binder), including the exact timing (before, with or after food) and the need to take them with food containing phosphate (including, for example, high-protein snacks).
- Take into account the person’s preferences on phosphate binders.
- If the person has problems taking the first phosphate binder offered, consider switching to the next recommended one (see recommendations below).
Phosphate Binders for Children and Young People
- Offer children and young people with CKD stage 4 or 5 and hyperphosphataemia a calcium-based phosphate binder to control serum phosphate levels.In August 2021, this was an off-label use of some calcium-based phosphate binders in people not on dialysis. See NICE’s information on prescribing medicines.
- If serum calcium increases towards, or above, the age-adjusted upper normal limit:
- investigate possible causes other than the phosphate binder
- consider reducing the dose of the calcium-based phosphate binder and adding sevelamer carbonate or switching to sevelamer carbonate alone.In August 2021, this was an off-label use of sevelamer carbonate. See NICE’s information on prescribing medicines.
- For all children and young people who are taking more than 1 phosphate binder, titrate the dosage to achieve the best possible control of serum phosphate while keeping serum calcium levels below the upper normal limit.
Phosphate Binders for Adults
First Phosphate Binder for Adults
- Offer adults with CKD stage 4 or 5 and hyperphosphataemia calcium acetate to control serum phosphate levels.In August 2021, this was an off-label use of calcium acetate in people not on dialysis. See NICE’s information on prescribing medicines.
- Offer sevelamer carbonate if calcium acetate is not indicated (for example, because of hypercalcaemia or low serum parathyroid hormone levels) or not tolerated.In August 2021, this was an off-label use of sevelamer carbonate. See NICE’s information on prescribing medicines.
- If calcium acetate and sevelamer carbonate cannot be used, consider:
- sucroferric oxyhydroxide, for adults on dialysis if a calcium-based phosphate binder is not needed or
- calcium carbonate, if a calcium-based phosphate binder is needed. In August 2021, this was an off-label use of these phosphate binders in people not on dialysis. See NICE’s information on prescribing medicines.
- Only consider lanthanum carbonate for adults with CKD stage 4 or 5 if other phosphate binders cannot be used.In August 2021, this was an off-label use of lanthanum carbonate phosphate binders in people not on dialysis and with serum phosphate levels less than 1.78 mmol/l. See NICE’s information on prescribing medicines.
Combinations of Phosphate Binders for Adults
- If adults with CKD stage 4 or 5 remain hyperphosphataemic after taking the maximum dose recommended in the BNF (or the maximum dose they can tolerate if that is lower), of a calcium-based phosphate binder:
- check they are taking it as prescribed
- consider combining a calcium-based phosphate binder with a non-calcium-based phosphate binder.
- For all adults who are taking more than 1 phosphate binder, titrate the dosage to achieve the best possible control of serum phosphate while keeping serum calcium levels below the upper normal limit.
Review of Treatments in Adults, Children and Young People
- At every routine clinical review, assess the person’s serum phosphate control, taking into account:
- diet
- whether they are taking the phosphate binders as prescribed
- other relevant factors, such as vitamin D levels, serum parathyroid hormone levels, alkaline phosphatase, serum calcium, medications that might affect serum phosphate, or dialysis.
Other Complications in Adults
Bone Metabolism and Osteoporosis
- Do not routinely measure calcium, phosphate, parathyroid hormone and vitamin D levels in adults with a GFR of 30 ml/min/1.73 m2 or more (GFR category G1, G2 or G3).
- Measure serum calcium, phosphate and parathyroid hormone concentrations in adults with a GFR of less than 30 ml/min/1.73 m2 (GFR category G4 or G5). Determine the subsequent frequency of testing by the measured values and the clinical circumstances. If doubt exists, seek specialist opinion.
- Offer bisphosphonates if indicated for the prevention and treatment of osteoporosis in adults with a GFR of 30 ml/min/1.73 m2 or more (GFR category G1, G2 or G3).
Vitamin D Supplements in the Management of CKD–Mineral and Bone Disorders
Detailed advice on the management of CKD–mineral and bone disorders is beyond the scope of this guideline. If uncertain, seek advice from your local renal service.- Do not routinely offer vitamin D supplementation to manage or prevent CKD–mineral and bone disorders.
- Offer colecalciferol or ergocalciferol to treat vitamin D deficiency in people with CKD and vitamin D deficiency.
- If vitamin D deficiency has been corrected and symptoms of CKD–mineral and bone disorders persist, offer alfacalcidol (1-alpha-hydroxycholecalciferol) or calcitriol (1-25-dihydroxycholecalciferol) to people with a GFR of less than 30 ml/min/1.73 m2 (GFR category G4 or G5).
- Monitor serum calcium and phosphate concentrations in people receiving alfacalcidol or calcitriol supplements.
Oral Bicarbonate Supplements in the Management of Metabolic Acidosis
Detailed advice on the management of metabolic acidosis is beyond the scope of this guideline. If uncertain, seek advice from your local renal service.- Consider oral sodium bicarbonate supplementation for adults with both:
- a GFR less than 30 ml/min/1.73 m2 (GFR category G4 or G5) and
- a serum bicarbonate concentration of less than 20 mmol/litre.

