Latest Guidance UpdatesMarch 2023: new recommendations on calcitonin gene-related peptide monoclonal antibodies and new safety advice on the use of therapies during pregnancy, in the section, Pharmacological Prevention. September 2022: new recommendations on the use of calcitonin-gene-related peptide monoclonal antibodies have been added to the section, Pharmacological Prevention. |
Overview
This updated Guidelines summary of SIGN 155 covers the pharmacological treatment and prevention options for migraines; recommendations on strategies to address medication overuse headache; sources of further information on migraine; and SIGN's migraine treatment pathway algorithm.
Recommendations are marked up with an (R) and good-practice points are marked up with a (✓); for further information about the ‘strength’ of recommendations, see the SIGN 155 Quick reference guideline.
Reflecting on your Learnings
Reflection is important for continuous learning and development, and a critical part of the revalidation process for UK healthcare professionals. Click here to access the Guidelines Reflection Record.
Treatment Pathway
Algorithm 1: Pharmacological Management of Patients with Migraine—Treatment Pathway
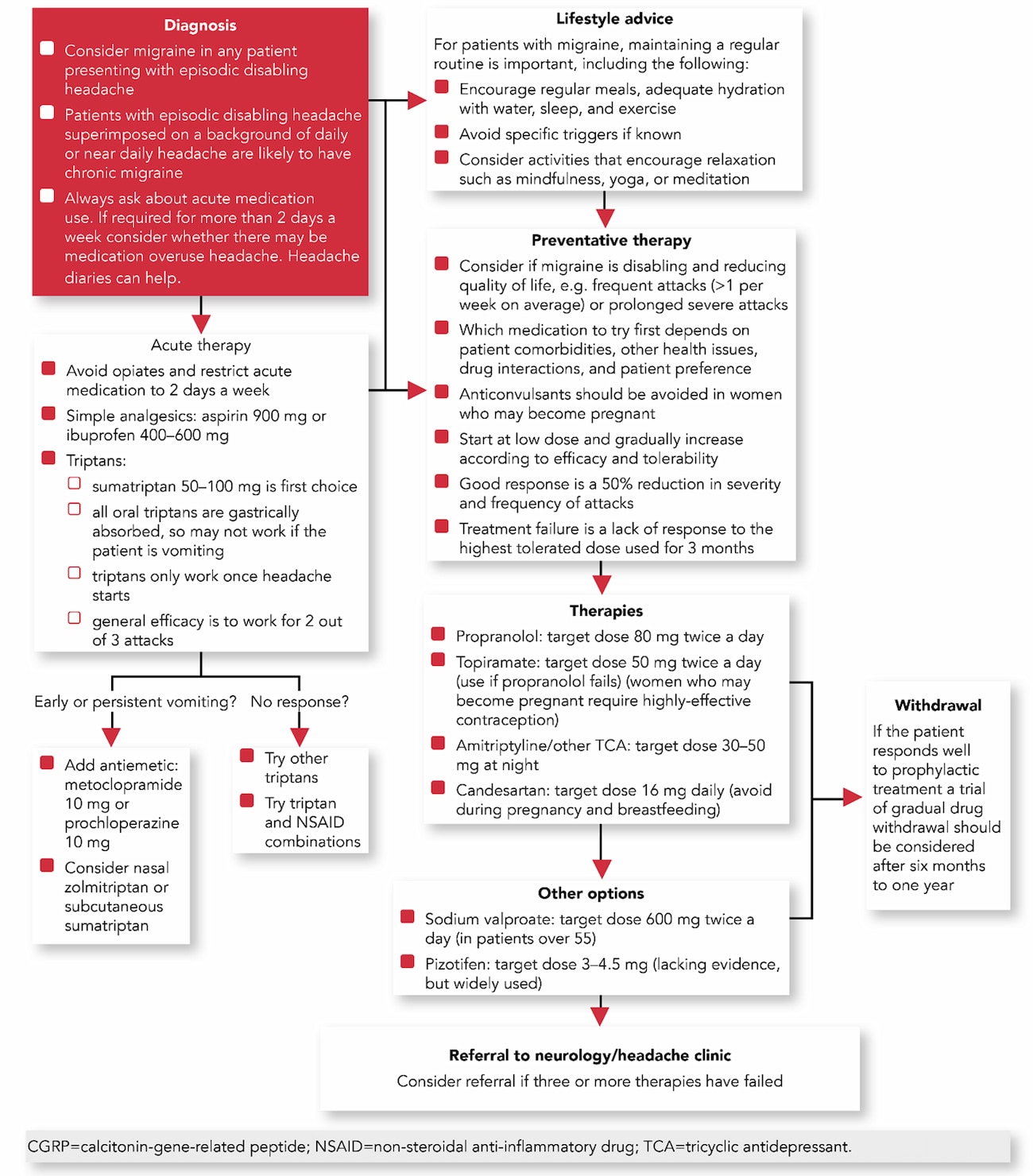
Treatment for Patients with Acute Migraine
- (✓) When starting acute treatment, healthcare professionals should warn patients about the risk of developing medication‑overuse headache.
Aspirin
- (R) Aspirin (900 mg) is recommended as first‑line treatment for patients with acute migraine
- (✓) Aspirin, in doses for migraine, is not an analgesic of choice during pregnancy and should not be used in the third trimester of pregnancy.
Non-steroidal Anti-inflammatory Drugs
- (R) Ibuprofen (400 mg) is recommended as first-line treatment for patients with acute migraine. If ineffective, the dose should be increased to 600 mg.
Paracetamol
- (R) Paracetamol (1000 mg) can be considered for treatment of patients with acute migraine who are unable to take other acute therapies
- Due to its safety profile, paracetamol is first choice for the short‑term relief of mild-to-moderate headache during any trimester of pregnancy. (✓)
Antiemetics
- (R) Metoclopramide (10 mg) or prochlorperazine (10 mg) can be considered in the treatment of headache in patients with acute migraine. They can be used either as an oral or parenteral formulation depending on presentation and setting
- (R) Metoclopramide (10 mg) or prochlorperazine (10 mg) should be considered for patients presenting with migraine-associated symptoms of nausea or vomiting. They can be used either as an oral or parenteral formulation depending on presentation and setting
- (✓) Metoclopramide should not be used regularly due to the risk of extrapyramidal side effects.
Triptans
- (R) Triptans are recommended as first‑line treatment for patients with acute migraine. The first choice is sumatriptan (50–100 mg), but others should be offered if sumatriptan fails
- (R) In patients with severe acute migraine or early vomiting, nasal zolmitriptan or subcutaneous sumatriptan should be considered
- (R) Triptans are recommended for the treatment of patients with acute migraine associated with menstruation
- (R) Sumatriptan can be considered for treatment of acute migraine in pregnant women in all stages of pregnancy. The risks associated with use should be discussed before commencing treatment.
Combination Therapies
- (R) Combination therapy using sumatriptan (50–85 mg) and naproxen (500 mg) should be considered for the treatment of patients with acute migraine.
Pharmacological Prevention
Beta Blockers
- (R) Propranolol (80–160 mg daily) is recommended as a first-line prophylactic treatment for patients with episodic or chronic migraine.
Topiramate
- (R) Topiramate (50–100 mg daily) is recommended as a prophylactic treatment for patients with episodic or chronic migraine
- (R) Before commencing treatment women should be informed of:
- the risks associated with taking topiramate during pregnancy
- the risk that potentially harmful exposure to topiramate may occur before a women is aware she is pregnant
- the need to use highly-effective contraception
- the need to seek further advice on migraine prophylaxis if pregnant or planning a pregnancy.
Tricyclic Antidepressants
- (R) Amitriptyline (25–150 mg at night) should be considered as a prophylactic treatment for patients with episodic or chronic migraine
- (R) In patients who cannot tolerate amitriptyline a less sedating tricyclic antidepressant should be considered.
Candesartan
- (R) Candesartan (16 mg daily) can be considered as a prophylactic treatment for patients with episodic or chronic migraine
- (R) Use of candesartan should be avoided during pregnancy and breastfeeding. Women using candesartan who are planning to become pregnant, or who are pregnant, should seek advice from their healthcare professional on switching to another therapy.
Sodium Valproate
- (R) Sodium valproate (400–1500 mg daily) can be considered as a prophylactic treatment for patients over the age of 55 with episodic or chronic migraine
- (R) Although valproate is not recommended for those under the age of 55, for those who remain on it and who fulfil Medicines and Healthcare products Regulatory Agency (MHRA) requirements, the safety advice is to inform the patient of the risks to children exposed to valproate in utero and the need to use effective contraception
- (R) If prescribing sodium valproate, check the MHRA website for current advice at www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency.
Calcium Channel Blockers
- (R) Flunarizine (10 mg daily) should be considered as a prophylactic treatment for patients with episodic or chronic migraine
- (R) Use of flunarazine should be avoided during pregnancy and breastfeeding. Women using flunarazine who are planning to become pregnant, or who are pregnant, should seek advice from their healthcare professional on switching to another therapy.
Gabapentin and Pregabalin
- There is a lack of evidence on the use of pregabalin in patients with episodic migraine
- (R) Gabapentin should not be considered as a prophylactic treatment for patients with episodic or chronic migraine.
Botulinum Toxin A
- (R) Botulinum toxin A is not recommended for the prophylactic treatment of patients with episodic migraine
- (R) Botulinum toxin A is recommended for the prophylactic treatment of patients with chronic migraine where medication overuse has been addressed and patients have been appropriately treated with three or more oral migraine prophylactic treatments
- (✓) Botulinum toxin A should only be administered by appropriately trained individuals under the supervision of a headache clinic or the local neurology service.
Calcitonin-gene-related Peptide Monoclonal Antibodies
- (R) Erenumab, fremanezumab, galcanezumab, and eptinezumab are recommended for the prophylactic treatment of patients with chronic migraine where medication overuse has been addressed and patients have not benefitted from appropriate trials of three or more oral migraine prophylactic treatments
- (R) Fremanezumab, galcanezumab, and eptinezumab can be considered for the prophylactic treatment of patients with episodic migraine where medication overuse has been addressed and patients have not benefitted from appropriate trials of three or more oral migraine prophylactic treatments
- (✓) Use of calcitonin-gene-related peptide (CGRP) monoclonal antibodies should only be initiated following consultation with a neurologist or headache specialist
- (✓) There should be careful consideration of potential risks and benefits to patients at high risk of ischaemic cardiovascular disease before prescribing CGRP monoclonal antibodies
- (✓) Use of CGRP monoclonal antibodies should be avoided during pregnancy and breastfeeding. A washout period of 6 months is advised before trying for a pregnancy
- (✓) Medication-overuse headache should be addressed before treatment with CGRPs. However, in patients where treatment of medication-overuse headache has been unsuccessful, CGRP monoclonal antibodies should still be considered.
Menstrual Migraine Prophylaxis
- (R) Frovatriptan (2.5 mg twice daily) should be considered as a prophylactic treatment in women with perimenstrual migraine from two days before until three days after bleeding starts
- (R) Zolmitriptan (2.5 mg three times daily) or naratriptan (2.5 mg twice daily) can be considered as alternatives to frovatriptan as prophylactic treatment in women with perimenstrual migraine from 2 days before until 3 days after bleeding starts
- (✓) Women with menstrual-related migraine who are using triptans at other times of the month should be advised that additional perimenstrual prophylaxis increases the risk of developing medication overuse headache.
Medication-Overuse Headache
- (R) In patients overusing acute treatment, medication overuse should be addressed
- (R) The choice of strategy to address medication overuse should be tailored to the individual patient and may be influenced by comorbidities. Strategies include:
- abrupt withdrawal alone and preventative treatment may then be considered after a delay
- abrupt withdrawal and immediately starting preventative treatment
- starting a preventative treatment without withdrawal
- (✓) Consider withdrawing regular opioids gradually
- (R) Prednisolone should not be used routinely in the management of patients with medication-overuse headache.

