Overview
This guideline sets out an antimicrobial prescribing strategy for adults, young people, children and babies aged 72 hours and over with a confirmed diagnosis of community-acquired pneumonia. It aims to optimise antibiotic use and reduce antibiotic resistance.
NB: NICE clinical guideline 191, Pneumonia in Adults: Diagnosis and Management, has been withdrawn by NICE during the COVID-19 pandemic. You are advised to use this NICE guidance summary. For recommendations on identifying and treating community-acquired bacterial pneumonia secondary to COVID-19, see our summary of NICE’s rapid guideline on managing COVID-19.
Read the related Guidelines in Practice article, Key Learning Points: NICE Community-acquired Pneumonia.
Pneumonia (Community-acquired)—Antimicrobial Prescribing
Algorithm 1: Pneumonia (Community-acquired)—Antimicrobial Prescribing
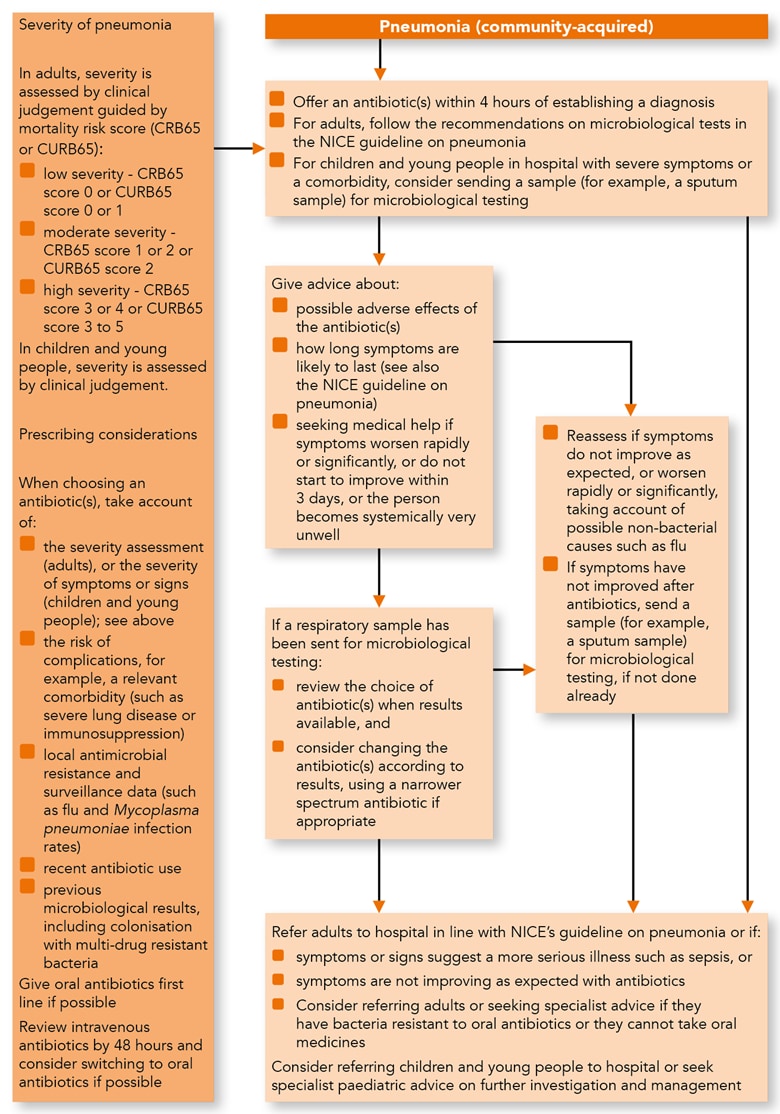
Managing Community-acquired Pneumonia
Treatment for Adults, Young People and Children
- Offer an antibiotic(s) for adults, young people and children with community- acquired pneumonia. When choosing an antibiotic (see the recommendations on choice of antibiotic), take account of:
- the severity assessment for adults, as set out in the NICE guideline on pneumonia in adults
- the severity of symptoms or signs[A] for children and young people, based on clinical judgement
- the risk of developing complications, for example, if the person has relevant comorbidity such as severe lung disease or immunosuppression
- local antimicrobial resistance and surveillance data (such as flu and Mycoplasma pneumoniae infection rates)
- recent antibiotic use
- recent microbiological results, including colonisation with multidrug-resistant bacteria
- Start antibiotic treatment as soon as possible after establishing a diagnosis of community-acquired pneumonia, and certainly within 4 hours (within 1 hour if the person has suspected sepsis and meets any of the high risk criteria for this—see the NICE guideline on sepsis).
- Give oral antibiotics first line if the person can take oral medicines, and the severity of their condition does not require intravenous antibiotics.
- If intravenous antibiotics are given, review by 48 hours and consider switching to oral antibiotics if possible.
- For adults with community-acquired pneumonia, follow the recommendations on microbiological tests in the NICE guideline on pneumonia in adults.
- For children and young people in hospital with community-acquired pneumonia, and severe symptoms or signs or a comorbidity, consider sending a sample (for example, sputum sample) for microbiological testing.
Advice
- Give advice to adults, young people and children with community-acquired pneumonia about:
- possible adverse effects of the antibiotic(s)
- how long symptoms are likely to last (see also the NICE guideline on pneumonia in adults)
- seeking medical help (if the person is receiving treatment in the community) if:
- symptoms worsen rapidly or significantly or
- symptoms do not start to improve within 3 days or
- the person becomes systemically very unwell.
Reassessment
- Reassess adults, young people and children with community-acquired pneumonia if symptoms or signs do not improve as expected or worsen rapidly or significantly.
- When reassessing adults, young people and children with community-acquired pneumonia, be aware of possible non-bacterial causes, such as flu.
- If a sample has been sent for microbiological testing:
- review the choice of antibiotic(s) when results are available and
- consider changing the antibiotic(s) according to results, using a narrower-spectrum antibiotic, if appropriate
- Send a sample (for example, a sputum sample) for microbiological testing if symptoms or signs have not improved following antibiotic treatment, and this has not been done already.
Referral and Seeking Specialist Advice
- Refer adults with community-acquired pneumonia to hospital as set out in the NICE guideline on pneumonia in adults, or if they have:
- any symptoms or signs suggesting a more serious illness or condition (for example, cardiorespiratory failure or sepsis) or
- symptoms that are not improving as expected with antibiotics
- Consider referring adults with community-acquired pneumonia to hospital, or seek specialist advice, if they:
- have bacteria that are resistant to oral antibiotics or
- cannot take oral medicines (exploring locally available options for giving intravenous antibiotics at home or in the community, rather than in hospital, if this is appropriate)
- Consider referring children and young people with community-acquired pneumonia to hospital, or seek specialist paediatric advice on further investigation and management.
Choice of Antibiotic
- When prescribing an antibiotic(s) for community-acquired pneumonia:
- follow Table 1 for adults aged 18 years and over
- follow Table 2 for children and young people under 18 years.
Table 1: Antibiotics for Adults Aged 18 Years and Over
| Antibiotic1 | Dosage and Course Length2 |
|---|---|
| First-choice Oral Antibiotic if Low Severity (Based on Clinical Judgement and Guided by CRB65 Score 0, or CURB65 Score 0 or 1)3 | |
| Amoxicillin | 500 mg 3 times a day (higher doses can be used – see BNF) for 5 days4 |
| Alternative Oral Antibiotics if Low Severity, for Penicillin Allergy or if Amoxicillin Unsuitable (for example, Atypical Pathogens Suspected5)3 | |
| Doxycycline | 200 mg on first day, then 100 mg once a day for 4 days (5‑day course in total)4 |
| Clarithromycin | 500 mg twice a day for 5 days4 |
| Erythromycin (in pregnancy) | 500 mg 4 times a day for 5 days4 |
| First-choice Oral Antibiotics if Moderate Severity (Based on Clinical Judgement and Guided by CRB65 Score 1 or 2, or CURB65 Score 2); Guided by Microbiological Results When Available3 | |
| Amoxicillin with (if Atypical Pathogens Suspected5): | 500 mg 3 times a day (higher doses can be used – see BNF) for 5 days4 |
| Clarithromycin6 or | 500 mg twice a day for 5 days4 |
| Erythromycin6 (in pregnancy) | 500 mg 4 times a day for 5 days4 |
| Alternative Oral Antibiotics if Moderate Severity, for Penicillin Allergy; Guided by Microbiological Results When Available3 | |
| Doxycycline | 200 mg on first day, then 100 mg once a day for 4 days (5‑day course in total)4 |
| Clarithromycin | 500 mg twice a day for 5 days4 |
| First-choice antibiotics if high severity (based on clinical judgement and guided by CRB65 score 3 or 4, or CURB65 score 3 to 5); guided by microbiological results when available3 | |
| Co‑amoxiclav with: | 500/125 mg 3 times a day orally or 1.2 g 3 times a day IV7 for 5 days4 |
| Clarithromycin or | 500 mg twice a day orally or IV7 for 5 days4 |
| Erythromycin (in pregnancy) | 500 mg 4 times a day orally for 5 days4 |
| Alternative Antibiotic if High Severity, for Penicillin Allergy; Guided by Microbiological Results When Available3 | |
| Levofloxacin8 (consider safety issues) | 500 mg twice a day orally or IV7 for 5 days5 |
| Consult local microbiologist if fluoroquinolone not appropriate. | |
| 1 See BNF for appropriate use and dosing in specific populations, for example, hepatic impairment, renal impairment, pregnancy and breastfeeding, and administering intravenous (or, where appropriate, intramuscular) antibiotics. 2 Oral doses are for immediate-release medicines. 3 Give oral antibiotics first line if the person can take oral medicines, and the severity of their condition does not require intravenous antibiotics. 4 Stop antibiotic treatment after 5 days unless microbiological results suggest a longer course is needed or the person is not clinically stable (fever in past 48 hours or more than 1 sign of clinical instability [systolic blood pressure <90 mmHg, heart rate >100/minute, respiratory rate >24/minute, arterial oxygen saturation <90% or PaO2 <60 mmHg in room air]). 5 Mycoplasma pneumoniae infection occurs in outbreaks approximately every 4 years. 6 Consider adding a macrolide to amoxicillin if atypical pathogens suspected. Review when microbiological results available. 7 Review intravenous antibiotics by 48 hours and consider switching to oral antibiotics if possible. 8 See Medicines and Healthcare products Regulatory Agency (MHRA) advice for restrictions and precautions for using fluoroquinolone antibiotics because of very rare reports of disabling and potentially long-lasting or irreversible side effects affecting musculoskeletal and nervous systems. Warnings include: stopping treatment at first signs of a serious adverse reaction (such as tendonitis), prescribing with special caution for people over 60 years and avoiding coadministration with a corticosteroid (March 2019). Abbreviations: BNF = British National Formulary; CRB65 = c onfusion, r espiratory rate ≥30/minute, low systolic [<90 mmHg] or diastolic [≤60 mmHg] b lood pressure, age ≥65; CURB65 = c onfusion, u rea >7 mmol/l, r espiratory rate ≥30/minute, low systolic [<90 mmHg] or diastolic [≤60 mmHg] b lood pressure, age ≥65; IV = intravenous; PaO2 = partial pressure of oxygen. | |
Table 2: Antibiotics for Children and Young People Under 18 Years
| Antibiotic1 | Dosage and Course Length2 |
|---|---|
| Children Under 1 Month | |
| Refer to paediatric specialist. | |
| Children Aged 1 Month and Over | |
| First-choice Oral Antibiotic if Non-severe Symptoms or Signs (Based on Clinical Judgement)3 | |
| Amoxicillin | 1 month to 11 months, 125 mg 3 times a day for 5 days4 1 year to 4 years, 250 mg 3 times a day for 5 days4 5 years to 17 years, 500 mg 3 times a day for 5 days4 (higher doses can be used for all ages – see BNF for children) |
| Alternative Oral Antibiotics if Non-severe Symptoms or Signs (Based on Clinical Judgement), for Penicillin Allergy or if Amoxicillin Unsuitable (for example, Atypical Pathogens Suspected5)3 | |
| Clarithromycin | 1 month to 11 years: Under 8 kg, 7.5 mg/kg twice a day for 5 days4 8 kg to 11 kg, 62.5 mg twice a day for 5 days4 12 kg to 19 kg, 125 mg twice a day for 5 days4 20 kg to 29 kg, 187.5 mg twice a day for 5 days4 30 kg to 40 kg, 250 mg twice a day for 5 days4 12 years to 17 years: 250 mg to 500 mg twice a day for 5 days4 |
| Erythromycin (in pregnancy) | 8 years to 17 years, 250 mg to 500 mg 4 times a day for 5 days4 |
| Doxycycline6 | 12 years to 17 years, 200 mg on first day, then 100 mg once a day for 4 days (5‑day course in total)4 |
| First-choice Antibiotic(s) if Severe Symptoms or Signs (Based on Clinical Judgement); Guided by Microbiological Results When Available3 | |
| Co‑amoxiclav | Oral doses: 1 month to 11 months, 0.5 ml/kg of 125/31 suspension 3 times a day for 5 days4 1 years to 5 years, 10 ml of 125/31 suspension 3 times a day or 0.5 ml/kg of 125/31 suspension 3 times a day for 5 days4,7 6 years to 11 years, 10 ml of 250/62 suspension 3 times a day or 0.3 ml/kg of 250/62 suspension 3 times a day for 5 days4 12 years to 17 years, 500/125 mg 3 times a day for 5 days4 IV doses8: 1 month to 2 months, 30 mg/kg 2 times a day4 3 months to 17 years, 30 mg/kg 3 times a day (maximum 1.2 g per dose 3 times a day)4 |
| With (if Atypical Pathogen Suspected5): | |
| Clarithromycin or | Oral doses: see above for clarithromycin; for 5 days4 IV doses8: 1 month to 11 years, 7.5 mg/kg twice a day (maximum 500 mg per dose)4 12 years to 17 years, 500 mg twice a day4 |
| Erythromycin (in pregnancy) | See oral dose above for erythromycin; for 5 days4 |
| Alternative Antibiotics if Severe Symptoms or Signs (Based on Clinical Judgement), for Penicillin Allergy; Guided by Microbiological Results When Available3 | |
| Consult local microbiologist. | |
| 1 See BNF for children for appropriate use and dosing in specific populations, for example, hepatic impairment, renal impairment, pregnancy and breastfeeding, and administering intravenous (or, where appropriate, intramuscular) antibiotics. 2 Oral doses are for immediate-release medicines. The age bands apply to children of average size and, in practice, the prescriber will use the age bands in conjunction with other factors such as the severity of the condition being treated and the child’s size in relation to the average size of children of the same age. 3 Give oral antibiotics first line if the person can take oral medicines, and the severity of their condition does not require intravenous antibiotics. 4 Stop antibiotic treatment after 5 days unless microbiological results suggest a longer course length is needed or the person is not clinically stable. 5 Mycoplasma pneumoniae infection occurs in outbreaks approximately every 4 years and is more common in school-aged children. 6 See BNF for children for use of doxycycline in children under 12. 7 Or 5 ml of 250/62 suspension. 8 Review intravenous antibiotics by 48 hours and consider switching to oral antibiotics if possible. Abbreviations: BNFC = British National Formulary for Children; IV = intravenous. | |
Footnotes
[A] At the time of publication (September 2019), no validated severity assessment tools are available for children and young people with community-acquired pneumonia, and severity of symptoms or signs should be based on clinical judgement.

