Overview
This Guidelines summary covers managing cellulitis and erysipelas and antimicrobial prescribing in primary care. The guideline sets out an antimicrobial prescribing strategy for adults, young people, children, and babies aged 72 hours and over with cellulitis and erysipelas. It aims to optimise antibiotic use and reduce antibiotic resistance. Refer to the full guideline for a complete set of recommendations.
Reflecting on your Learnings
Reflection is important for continuous learning and development, and a critical part of the revalidation process for UK healthcare professionals. Click here to access the Guidelines Reflection Record.
Visual Summary
Algorithm 1: Cellulitis and Eryspielas—Antimicrobial Prescribing
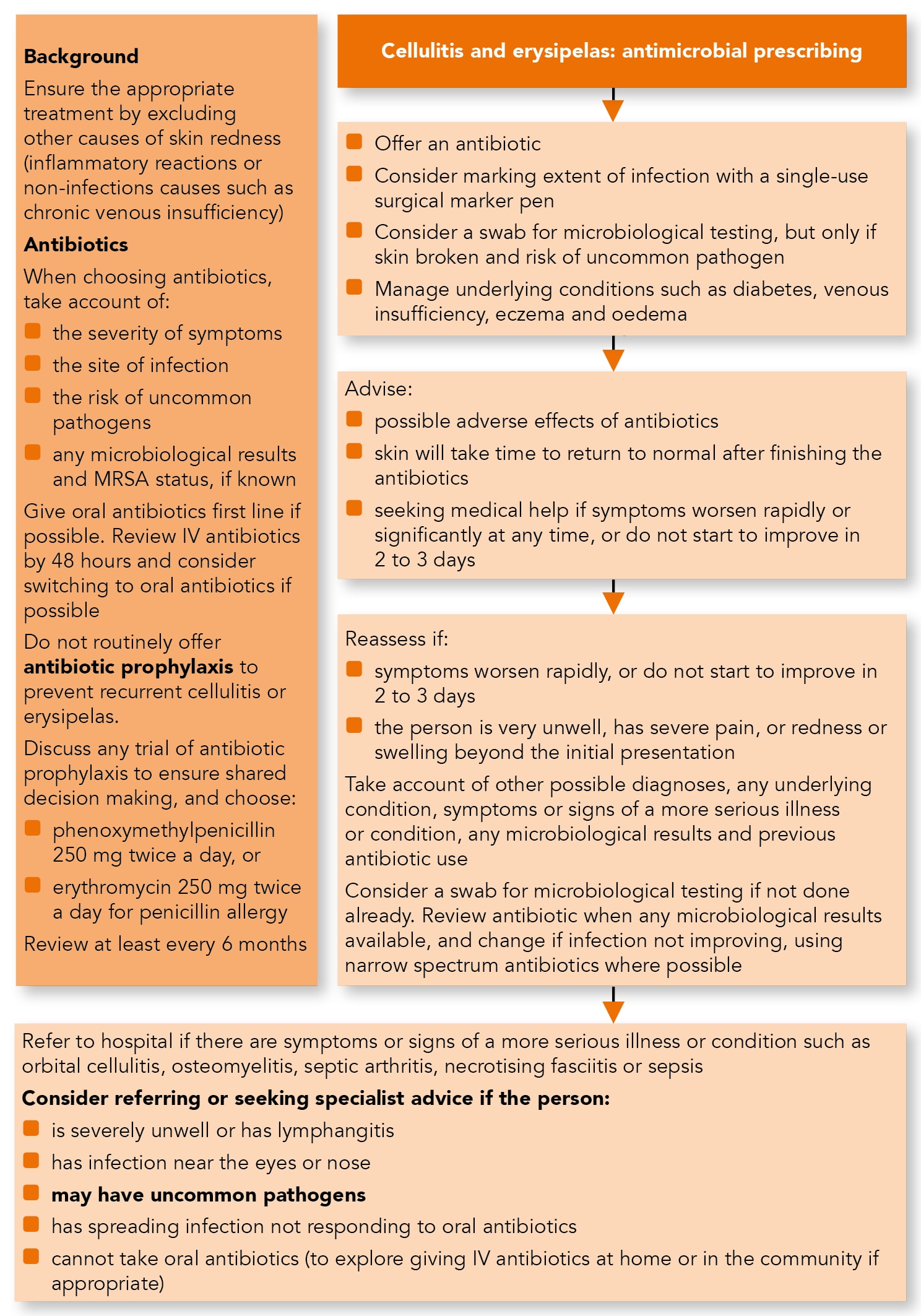
Managing Cellulitis and Erysipelas
Treatment
- To ensure that cellulitis and erysipelas are treated appropriately, exclude other causes of skin redness such as:
- an inflammatory reaction to an immunisation or an insect bite or
- a non-infectious cause such as chronic venous insufficiency.
- Consider taking a swab for microbiological testing from people with cellulitis or erysipelas to guide treatment, but only if the skin is broken and:
- there is a penetrating injury or
- there has been exposure to water-borne organisms or
- the infection was acquired outside the UK.
- Before treating cellulitis or erysipelas, consider drawing around the extent of the infection with a single-use surgical marker pen to monitor progress. Be aware that redness may be less visible on darker skin tones.
- Offer an antibiotic for people with cellulitis or erysipelas. When choosing an antibiotic (see Table 1), take account of:
- the severity of symptoms
- the site of infection (for example, near the eyes or nose)
- the risk of uncommon pathogens (for example, from a penetrating injury, after exposure to water-borne organisms, or an infection acquired outside the UK)
- previous microbiological results from a swab
- the person’s meticillin-resistant Staphylococcus aureus (MRSA) status if known.
- Give oral antibiotics first line if the person can take oral medicines, and the severity of their condition does not require intravenous antibiotics.
- If intravenous antibiotics are given, review by 48 hours and consider switching to oral antibiotics if possible.
- Manage any underlying condition that may predispose to cellulitis or erysipelas, for example:
- diabetes
- venous insufficiency
- eczema
- oedema, which may be an adverse effect of medicines such as calcium channel blockers.
Advice
- When prescribing antibiotics for cellulitis or erysipelas, give advice about:
- possible adverse effects of antibiotics
- the skin taking some time to return to normal after the course of antibiotics has finished
- seeking medical help if symptoms worsen rapidly or significantly at any time, or do not start to improve within 2 to 3 days.
Reassessment
- Reassess people with cellulitis or erysipelas if symptoms worsen rapidly or significantly at any time, do not start to improve within 2 to 3 days, or the person:
- becomes systemically very unwell or
- has severe pain out of proportion to the infection or
- has redness or swelling spreading beyond the initial presentation (taking into account that some initial spreading may occur, and that redness may be less visible on darker skin tones).
- When reassessing people with cellulitis or erysipelas, take account of:
- other possible diagnoses, such as an inflammatory reaction to an immunisation or an insect bite, gout, superficial thrombophlebitis, eczema, allergic dermatitis or deep vein thrombosis
- any underlying condition that may predispose to cellulitis or erysipelas, such as oedema, diabetes, venous insufficiency or eczema
- any symptoms or signs suggesting a more serious illness or condition, such as lymphangitis, orbital cellulitis, osteomyelitis, septic arthritis, necrotising fasciitis or sepsis
- any results from microbiological testing
- any previous antibiotic use, which may have led to resistant bacteria.
- Consider taking a swab for microbiological testing from people with cellulitis or erysipelas if the skin is broken and this has not been done already.
- If a swab has been sent for microbiological testing:
- review the choice of antibiotic(s) when results are available and
- change the antibiotic(s) according to results if symptoms or signs of the infection are not improving, using a narrow-spectrum antibiotic if possible.
Referral and Seeking Specialist Advice
- Refer people to hospital if they have any symptoms or signs suggesting a more serious illness or condition, such as orbital cellulitis, osteomyelitis, septic arthritis, necrotising fasciitis or sepsis.
- Consider referring people with cellulitis or erysipelas to hospital, or seek specialist advice, if they:
- are severely unwell or
- have infection near the eyes or nose (including periorbital cellulitis) or
- could have uncommon pathogens, for example, after a penetrating injury, exposure to water-borne organisms, or an infection acquired outside the UK or
- have spreading infection that is not responding to oral antibiotics or
- lymphangitis or
- cannot take oral antibiotics (exploring locally available options for giving intravenous antibiotics at home or in the community, rather than in hospital, where appropriate).
Choice of Antibiotic
When prescribing an antibiotic for cellulitis or erysipelas, follow:- table 1 for adults aged 18 years and over
- table 2 for children and young people under 18 years.
Table 1: Antibiotics for Adults Aged 18 Years and Over
| Antibiotic[A] | Dosage and course length[B] | |
|---|---|---|
| First-choice antibiotic (give oral unless person unable to take oral or severely unwell)[C] | ||
| Flucloxacillin | 500 mg to 1 g four times a day orally[D] for 5 to 7 days[E] | or 1 to 2 g four times a day IV[F] |
| Alternative first-choice antibiotics for penicillin allergy or if flucloxacillin unsuitable (give oral unless person unable to take oral or severely unwell)[C] | ||
| Clarithromycin | 500 mg twice a day orally for 5 to 7 days[E] | or 500 mg twice a day IV[F] |
| Erythromycin (in pregnancy) | 500 mg four times a day orally for 5 to 7 days[E] | |
| Doxycycline | 200 mg on first day, then 100 mg once a day orally for 5 to 7 days in total[E] | |
| First-choice antibiotic if infection near the eyes or nose[G] (consider seeking specialist advice; give oral unless person unable to take oral or severely unwell)[C] | ||
| Co-amoxiclav | 500/125 mg three times a day orally for 7 days[E] | or 1.2 g three times a day IV[F] |
| Alternative first-choice antibiotics if infection near the eyes or nose[G] for penicillin allergy or if co‑amoxiclav unsuitable (consider seeking specialist advice; give oral unless person unable to take oral or severely unwell)[C] | ||
| Clarithromycin | 500 mg twice a day orally for 7 days[E] | or 500 mg twice a day IV[F] |
| with metronidazole | 400 mg three times a day orally for 7 days[E] | or 500 mg three times a day IV[F] |
| Alternative choice antibiotics for severe infection | ||
| Co-amoxiclav | 500/125 mg three times a day orally for 7 days[E] | or 1.2 g three times a day IV[F] |
| Cefuroxime | 750 mg to 1.5 g three or four times a day IV[F] | |
| Clindamycin | 150 to 300 mg four times a day (can be increased to 450 mg four times a day) orally for 7 days[E] | or 600 mg to 2.7 g daily IV in two to four divided doses, increased if necessary in life-threatening infection to 4.8 g daily (maximum per dose 1.2 g)[F] |
| Ceftriaxone (only for ambulatory care[H]) | 2 g once a day IV[F] | |
| Antibiotics to be added if MRSA infection is suspected or confirmed (combination therapy with an antibiotic listed above)[H] | ||
| Vancomycin[I,J] | 15 to 20 mg/kg two or three times a day IV (maximum 2 g per dose), adjusted according to serum vancomycin concentration[F] | |
| Teicoplanin[I,J] | Initially 6 mg/kg every 12 hours for three doses, then 6 mg/kg once a day IV[F] | |
| Linezolid (if vancomycin or teicoplanin cannot be used; specialist use only)[J] | 600 mg twice a day orally | or 600 mg twice a day IV[F] |
| [A] See BNF for appropriate use and dosing in specific populations, for example, hepatic impairment, renal impairment, pregnancy and breastfeeding, and administering intravenous (or, where appropriate, intramuscular) antibiotics. [B] Oral doses are for immediate-release medicines. [C] Give oral antibiotics first line if the person can take oral medicines, and the severity of their symptoms does not require intravenous antibiotics. [D] The upper dose of 1 g four times a day would be off‑label. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Good practice in prescribing and managing medicines and devices for further information. [E] A longer course (up to 14 days in total) may be needed based on clinical assessment. However, skin does take some time to return to normal, and full resolution of symptoms at 5 to 7 days is not expected. [F] If intravenous antibiotics are given, review by 48 hours and consider switching to oral antibiotics if possible for the appropriate course length. [G] Infection around the eyes or the nose (the triangle from the bridge of the nose to the corners of the mouth, or immediately around the eyes including periorbital cellulitis) is of more concern because of risk of a serious intracranial complication. [H] Other antibiotics may be appropriate based on microbiological results and specialist advice. [I] See BNF for information on therapeutic drug monitoring. [J] See BNF for information on monitoring of patient parameters. | ||
| Abbreviations: BNF=British National Formulary; IV=intravenous; MRSA=meticillin-resistant Staphylococcus aureus. | ||
Table 2: Antibiotics for Children and Young People Under 18 Years
| Antibiotic[A] | Dosage and course length[B] | |
|---|---|---|
| Children under 1 month | ||
| Antibiotic choice based on specialist advice | ||
| Children aged 1 month and over | ||
| First-choice antibiotic (give oral unless person unable to take oral or severely unwell)[C] | ||
| Flucloxacillin[D] | 1 month to 1 year, 62.5 mg to 125 mg four times a day orally for 5 to 7 days[E] 2 to 9 years, 125 mg to 250 mg four times a day orally for 5 to 7 days[E] 10 to 17 years, 250 mg to 500 mg four times a day orally for 5 to 7 days[E] | or 1 month to 17 years, 12.5 mg to 25 mg/kg four times a day IV (maximum 1 g four times a day)[F] |
| Alternative first-choice antibiotics for penicillin allergy or if flucloxacillin unsuitable (give oral unless person unable to take oral or severely unwell)[C] | ||
| Co-amoxiclav (not in penicillin allergy)[G] | 1 to 11 months, 0.25 ml/kg of 125/31 suspension three times a day orally for 5 to 7 days[E] (dose doubled in severe infection) 1 to 5 years, 0.25 ml/kg or 5 ml of 125/31 suspension three times a day orally for 5 to 7 days[E] (dose doubled in severe infection) 6 to 11 years, 0.15 ml/kg or 5 ml of 250/62 suspension three times a day orally for 5 to 7 days[E] (dose doubled in severe infection) 12 to 17 years, 250/125 mg or 500/125 mg three times a day orally for 5 to 7 days[E] | or 1 to 2 months, 30 mg/kg twice a day IV[F] 3 months to 17 years, 30 mg/kg three times a day IV (maximum 1.2 g three times a day)[F] |
| Clarithromycin | 1 month to 11 years: Under 8 kg, 7.5 mg/kg twice a day orally for 5 to 7 days[E] 8 to 11 kg, 62.5 mg twice a day orally for 5 to 7 days[E] 12 to 19 kg, 125 mg twice a day orally for 5 to 7 days[E] 20 to 29 kg, 187.5 mg twice a day orally for 5 to 7 days[E] 30 to 40 kg, 250 mg twice a day orally for 5 to 7 days[E] 12 to 17 years: 250 to 500 mg twice a day orally for 5 to 7 days[E] | or 1 month to 11 years, 7.5 mg/kg twice a day IV (maximum 500 mg per dose)[F] 12 to 17 years, 500 mg twice a day IV[F] |
| Erythromycin (in pregnancy) | 8 to 17 years, 250 to 500 mg four times a day orally for 5 to 7 days[E] | |
| First-choice antibiotic if infection near the eyes or nose[H] (consider seeking specialist advice; give oral unless person unable to take oral or severely unwell)[C] | ||
| Co-amoxiclav[G] | 1 to 11 months, 0.25 ml/kg of 125/31 suspension three times a day orally for 7 days[E] (dose can be doubled in severe infection) 1 to 5 years, 0.25 ml/kg or 5 ml of 125/31 suspension three times a day orally for 7 days[E] (dose can be doubled in severe infection) 6 to 11 years, 0.15 ml/kg or 5 ml of 250/62 suspension three times a day orally for 7 days[E] (dose can be doubled in severe infection) 12 to 17 years, 250/125 mg or 500/125 mg three times a day orally for 7 days[E] | or 1 to 2 months, 30 mg/kg twice a day IV[F] 3 months to 17 years, 30 mg/kg three times a day IV (maximum 1.2 g three times a day)[F] |
| Alternative first-choice antibiotics if infection near the eyes or nose[H] for penicillin allergy or if co‑amoxiclav unsuitable (consider seeking specialist advice; give oral unless person unable to take oral or severely unwell)[C] | ||
| Clarithromycin | 1 month to 11 years: Under 8 kg, 7.5 mg/kg twice a day orally for 7 days[E] 8 to 11 kg, 62.5 mg twice a day orally for 7 days[E] 12 to 19 kg, 125 mg twice a day orally for 7 days[E] 20 to 29 kg, 187.5 mg twice a day orally for 7 days[E] 30 to 40 kg, 250 mg twice a day orally for 7 days[E] 12 to 17 years: 250 to 500 mg twice a day orally for 7 days[E] | or 1 month to 11 years, 7.5 mg/kg twice a day IV (maximum 500 mg per dose)[F] 12 to 17 years, 500 mg twice a day IV[F] |
| with (if anaerobes suspected): Metronidazole | 1 month, 7.5 mg/kg twice a day orally for 7 days[E] 2 months to 11 years, 7.5 mg/kg three times a day orally (maximum per dose 400 mg) for 7 days[E] 12 to 17 years, 400 mg three times a day for 7 days[E] | or 1 month, loading dose 15 mg/kg, then (after 8 hours) 7.5 mg/kg three times a day IV[F] 2 months to 17 years, 7.5 mg/kg three times a day IV (maximum per dose 500 mg)[F] |
| Alternative choice antibiotics for severe infection[I] | ||
| Co-amoxiclav[G] | 1 to 11 months, 0.25 ml/kg of 125/31 suspension three times a day orally for 7 days[E] (dose can be doubled) 1 to 5 years, 0.25 ml/kg or 5 ml of 125/31 suspension three times a day orally for 7 days[E] (dose can be doubled) 6 to 11 years, 0.15 ml/kg or 5 ml of 250/62 suspension three times a day orally for 7 days[F] (dose can be doubled) 12 to 17 years, 250/125 mg or 500/125 mg three times a day orally for 7 days[E] | or 1 to 2 months, 30 mg/kg twice a day IV[F] 3 months to 17 years, 30 mg/kg three times a day IV (maximum 1.2 g three times a day)[F] |
| Cefuroxime | 1 month to 17 years, 20 mg/kg three times a day IV (maximum 750 mg per dose), can be increased to 50 to 60 mg/kg three or four times a day IV (maximum 1.5 g per dose)[F] | |
| Clindamycin | 1 month to 17 years, 3 to 6 mg/kg four times a day orally (maximum per dose 450 mg) for 7 days[E] | or 1 month to 17 years, 3.75 to 6.25 mg/kg four times a day IV, increased if necessary, in life-threatening infection to 10 mg/kg four times a day IV (maximum per dose 1.2 g); total daily dose may alternatively be given in three divided doses (maximum per dose 1.2 g)[F] |
| Antibiotics to be added if suspected or confirmed MRSA infection (combination therapy with an antibiotic listed above)[I] | ||
| Vancomycin[J,K] | 1 month to 11 years, 10 to 15 mg/kg four times a day IV, adjusted according to serum vancomycin concentration[F] 12 to 17 years, 15 to 20 mg/kg two or three times a day IV (maximum 2 g per dose), adjusted according to serum vancomycin concentration[F] | |
| Teicoplanin[J,K] | 1 month, initially 16 mg/kg for one dose, then (after 24 hours) 8 mg/kg once a day IV[F] 2 months to 11 years, initially 10 mg/kg every 12 hours for 3 doses, then 6 to 10 mg/kg once a day IV[F] 12 to 17 years, initially 6 mg/kg every 12 hours for three doses, then 6 mg/kg once a day IV[F] | |
| Linezolid (if vancomycin or teicoplanin cannot be used; specialist use only)[K,L] | 1 month to 11 years, 10 mg/kg three times a day orally (maximum 600 mg per dose) 12 to 17 years, 600 mg twice a day orally | or 1 month to 11 years, 10 mg/kg three times a day IV (maximum 600 mg per dose)[F] 12 to 17 years, 600 mg twice a day IV[F] |
| [A] See BNF for children for appropriate use and dosing in specific populations, for example, hepatic impairment, renal impairment, pregnancy and breastfeeding, and administering intravenous (or, where appropriate, intramuscular) antibiotics. [B] The age bands apply to children of average size and, in practice, the prescriber will use the age bands in conjunction with other factors such as the severity of the condition and the child’s size in relation to the average size of children of the same age. Oral doses are for immediate-release medicines. [C] Give oral antibiotics first line if the child or young person can take oral medicines, and the severity of their symptoms does not require intravenous antibiotics. [D] If flucloxacillin oral solution is not tolerated because of poor palatability, consider capsules (see Medicines for Children leaflet on helping your child to swallow tablets). [E] A longer course (up to 14 days in total) may be needed based on clinical assessment. However, skin does take some time to return to normal, and full resolution of symptoms at 5 to 7 days is not expected. [F] If intravenous antibiotics are given, review by 48 hours and consider switching to oral antibiotics if possible for the appropriate course length. [G] Co-amoxiclav 400/57 suspension may also be considered to allow twice daily dosing (see BNF for children for dosing information). [H] Infection around the eyes or the nose (the triangle from the bridge of the nose to the corners of the mouth, or immediately around the eyes including periorbital cellulitis) is of more concern because of risk of a serious intracranial infection. [I] Other antibiotics may be appropriate based on microbiological results and specialist advice. [J] See BNF for children for information on therapeutic drug monitoring. [K] See BNF for children for information on monitoring of patient parameters. [L] Not licensed in children and young people under 18 years, so use would be off‑label. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Good practice in prescribing and managing medicines and devices for further information. | ||
| Abbreviations: BNF=British National Formulary; IV=intravenous; MRSA=meticillin-resistant Staphylococcus aureus. | ||

