Overview
This Guidelines summary covers key recommendations from national guidance on lipid management for the primary and secondary prevention of cardiovascular disease (CVD), including:
- treatment pathway for primary and secondary prevention of CVD
- management
- primary prevention risk assessment
- special patient populations
- extent of lipid lowering with available therapies
- monitoring
- titration threshold/targets
- specialist services
- triglycerides
- statin intolerance.
Lipid Management Pathway
Algorithm 1: Lipid Management Pathway
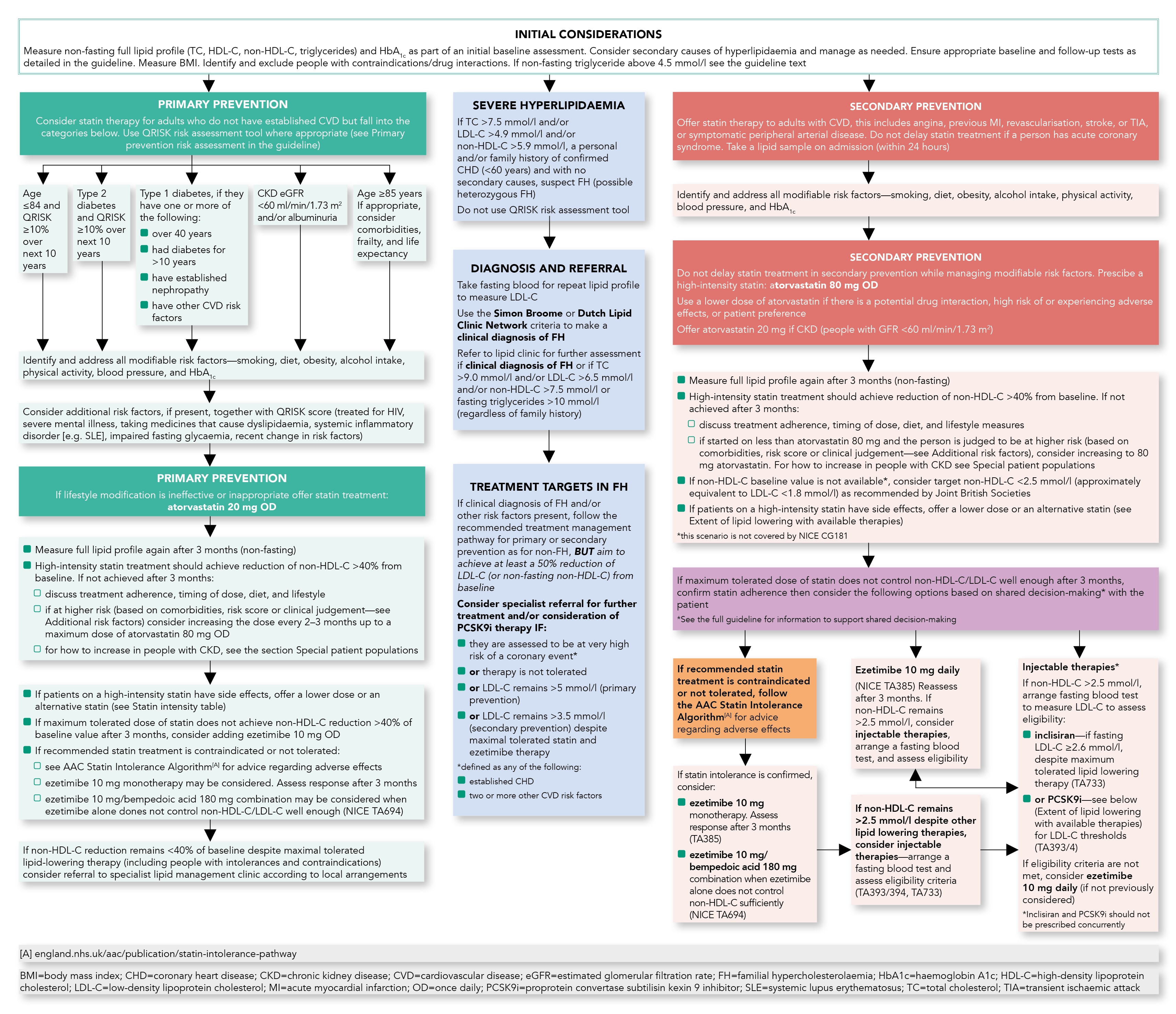
For information on specialist services, refer to the full guideline.
Management
This guidance applies to new patients and may also be taken into consideration for those already on statins at their annual review.- If 40% reduction of non-high-density lipoprotein cholesterol (non-HDL-C) is not achieved, offer high-intensity statins
- Discuss with people who are stable on a low- or medium-intensity statin the likely benefits and potential risk of side effects if changed to a high-intensity statin when they have a medication review and agree with the person whether a change is needed
- Ezetimibe, alirocumab, evolocumab, or inclisiran can be added when patients’ low-density lipoprotein cholesterol (LDL-C) levels are not lowered enough with the maximally tolerated dose of statins
- Bempedoic acid with ezetimibe is an option when statins are contraindicated or not tolerated, and when ezetimibe alone does not control LDL-C well enough
- Do not offer a fibrate, nicotinic acid, bile acid binder, or omega-3 fatty acids alone or in combination with statin, for the prevention of CVD. Check the NICE guideline on Cardiovascular disease: risk assessment and reduction, including lipid modification for exceptions.
Primary Prevention Risk Assessment
- QRISK3 is the current version of the QRISK calculator
- do not use this risk assessment tool for people with established CVD or those who are at high risk of developing CVD because of familial hypercholesterolaemia or other inherited disorders of lipid metabolism
- do not use a risk assessment tool to assess CVD risk in people with type 1 diabetes, or estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2 and/or albuminuria
- consider people aged 85 or over at increased risk of CVD because of age alone, particularly people who smoke or have raised blood pressure.
Additional Risk Factors
- Standard CVD risk scores including QRISK may underestimate risk in people who have additional risk because of underlying medical conditions or treatments. These groups include the following groups of people:
- severe obesity (body mass index over 40 kg/m2) increases CVD risk
- treated for HIV
- serious mental health problems
- taking medicines that can cause dyslipidaemia such as antipsychotic medication, corticosteroids, or immunosuppressant drugs
- autoimmune disorders such as systemic lupus erythematosus, and other systemic inflammatory disorders
- non-diabetic hyperglycaemia
- significant hypertriglyceridaemia (fasting triglycerides 4.5–9.9 mmol per litre)
- recent risk factor changes, for example, quit smoking, blood pressure, or lipid treatment
- Consider socioeconomic status as an additional factor contributing to CVD risk
- If QRISK is less than 10% over the next 10 years, give lifestyle advice and ensure regular review of CVD risk in line with guidance.
Special Patient Populations
Type 1 Diabetes
- While NICE recommends offering statins to patients with type 1 diabetes as detailed in the algorithm, it also states to consider statins in all adults with type 1 diabetes.
Chronic Kidney Disease
- Offer atorvastatin 20 mg for the primary or secondary prevention of CVD to people with chronic kidney disease (eGFR less than 60 ml/min/1.73 m2 and/or albuminuria)
- Increase the dose if a greater than 40% reduction in non-HDL-C is not achieved and eGFR is 30 ml/min/1.73 m2 or more
- Agree the use of higher doses with a renal specialist if eGFR is less than 30 ml/min/1.73 m2.
Extent of Lipid Lowering With Available Therapies
Table 1: Extent of Lipid Lowering With Available Therapies
| Approximate Reduction in LDL-C | |||||
| Statin Dose mg/day | 5 | 10 | 20 | 40 | 80 |
| Fluvastatin | 21%[A] | 27%[A] | 33%[B] | ||
| Pravastatin | 20%[A] | 24%[A] | 29%[A] | ||
| Simvastatin | 27%[A] | 32%[B] | 37%[B] | 42%[D] | |
| Atorvastatin | 37%[B] | 43%[C] | 49%[C] | 55%[C] | |
| Rosuvastatin | 38%[B] | 43%[C] | 48%[C] | 53%[C] | |
| Atorvastatin + ezetimibe 10 mg | 52%[C] | 54%[C] | 57%[C] | 61%[C] | |
| [A] Low-intensity statins—will produce an LDL-C reduction of 20–30% [B] Medium-intensity statins—will produce an LDL-C reduction of 31–40% [C] High-intensity statins—will produce an LDL-C reduction above 40% [D] Simvastatin 80 mg is not recommended due to muscle toxicity | |||||
| LDL-C=low-density lipoprotein cholesterol | |||||
- Rosuvastatin may be used as an alternative to atorvastatin if compatible with other drug therapy. Some people may need a lower starting dose (see British National Formulary)
- Low/medium intensity statins should only be used in intolerance or drug interactions
- Ezetimibe when combined with any statin is likely to give greater reduction in non-HDL-C/LDL-C than doubling the dose of the statin
- PCSK9i alone, or in combination with statins or ezetimibe, produces an additional LDL-C reduction of approximately 50% (range 25–70%)
- Bempedoic acid when combined with ezetimibe produces an additional LDL-C reduction of approximately 28% (range 22–33%), but no clinical outcome evidence is currently available
- Inclisiran alone, or in combination with statins or ezetimibe, produces an additional LDL-C reduction of approximately 50% (range 48–52%), but no clinical outcome evidence is currently available.
Monitoring
Baseline Measurements
- In addition to full lipid profile, measure renal, thyroid, and liver profiles (including albumin) and HbA1c to exclude secondary causes and comorbidities
- Measure baseline liver transaminase (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) before starting a statin
- Measure creatine kinase (CK) if unexplained muscle pain before starting a statin. CK should not be measured routinely, especially if a patient is asymptomatic.
Table 2: Baseline Measurements Table
| Primary Prevention | Secondary Prevention | |||
| Lipid profile | ALT or AST | Lipid profile | ALT or AST | |
| Baseline | ✓ | ✓ | ✓ | ✓ |
| 3 months | ✓ | ✓ | ✓ | ✓ |
| 6–9 months | If <40% non-HDL-C reduction, up titration required. Repeat full lipid profile and ALT or AST within 3 months of each up-titration of statin dose or addition of ezetimibe as required | |||
| 12 months | ✓ | ✓ | ✓ | ✓ |
| Yearly | ✓[A] | ✓[A] | ||
| Provide annual medication reviews for people taking statins to discuss effectiveness of therapy, medicines adherence, lifestyle modification and address CVD risk factors. [A] Consider an annual non-fasting full lipid profile to inform the discussion around effectiveness of lipid-lowering therapy, and any medicines non-adherence | ||||
| ALT=alanine aminotransferase; AST=aspartate aminotransferase; non-HDL-C=non-high-density lipoprotein cholesterol | ||||
Monitoring
- Repeat full lipid profile is non-fasting
- Measure liver transaminase within 3 months of starting treatment, then within 3 months of every additional up titration, and then again at 12 months, but not again unless clinically indicated
- If ALT or AST are greater than three times the upper limit of normal, do not initiate a statin, or discontinue statin therapy already prescribed. Repeat the liver function tests in a month
- If ALT or AST are elevated, but are less than three times the upper limit of normal, then:
- continue the statin and repeat in a month
- if they remain elevated but are less than three times the upper limit of normal then continue statin and repeat again in 6 months.
Titration Threshold/Targets
Table 3: Titration Thresholds
| NICE Titration Threshold | JBS3 | |
| Primary Prevention | Intensify lipid-lowering therapy if non-HDL-C reduction from baseline is <40% | Non-HDL-C <2.5 mmol/l (LDL-C <1.8 mmol/l) |
| Secondary Prevention | ||
| Intensify lipid-lowering therapy if non-HDL-C reduction from baseline is <40% | Non-HDL-C <2.5 mmol/l (LDL-C <1.8 mmol/l) | |
| FH | Optimise lipid-lowering therapy to achieve at least 50% reduction in LDL-C (or non-HDL-C) | |
| If baseline cholesterol is unknown in the setting of secondary prevention, use the Joint British Societies’ JBS3 consensus recommendation. Non-HDL-C=TC minus HDL-C; LDL-C=non-HDL-C minus (fasting triglycerides[A]/2.2) [A] Valid only when fasting triglycerides are less than 4.5 mmol/l | ||
| FH=familial hypercholesterolaemia; LDL-C=low-density lipoprotein cholesterol; non-HDL-C=non-high-density lipoprotein cholesterol | ||
Specialist Services
- The scope of specialist services available locally may include:
- lipid clinic
- PCSK9i clinic (offering initiation and subsequent follow up)
- familial hypercholesterolaemia genetic diagnosis and cascade testing
- lipoprotein apheresis service
- NICE eligibility criteria for PCSK9i and fasting LDL-C thresholds are summarised below in Table 4.
Table 4: Eligibility Criteria for PCSK9i and Fasting LDL-C Thresholds
| Without CVD | With CVD | ||
| High risk[A] | Very high risk[B] | ||
| Primary non-FH or mixed dyslipidaemia | Not recommended | LDL-C >4.0 mmol/l | LDL-C >3/5 mmol/l |
| Primary heterozygous FH | LDL-C >5.0 mmol/l | LDL-C >3.5 mmol/l | |
| See NICE TA393 on alirocumab and NICE TA394 on evolocumab. [A] History of any of the following: ACS; coronary or other arterial revascularisation procedures; CHD; ischaemic stroke; PAD [B] Recurrent CV events or CV events in more than one vascular bed (i.e. polyvascular disease) | |||
| ACS=acute coronary syndrome; CHD=coronary heart disease; CV=cardiovascular; CVD=cardiovascular disease; FH=familial hypercholesterolaemia; LDL-C=low-density lipoprotein cholesterol; PAD=peripheral arterial disease | |||
- Bempedoic acid/ezetimibe and inclisiran are available in primary care and do not require inititiation by specialist services
- PCSK9i may be available for prescribing in primary care; see local initiation pathways.
Triglycerides
Table 5: Triglyceride Concentrations
| Triglyceride Concentration | Action |
| >20 mmol/l | Refer to lipid clinic for urgent specialist review if not a result of excess alcohol or poor glycaemic control. At risk of acute pancreatitis |
| 10–20 mmol/l | Repeat the triglyceride measurement with a fasting test (after an interval of 5 days, but within 2 weeks) and review for potential secondary causes of hyperlipidaemia. Seek specialist advice if the triglyceride concentration remains >10 mmol/l. At risk of acute pancreatitis |
| 4.5–9.9 mmol/l | If non-fasting triglycerides >4.5 mmol/l, repeat with a fasting triglyceride measurementBe aware that the CVD risk may be underestimated by risk assessment tools, optimise the management of other CVD risk factors present, and seek specialist advice if non-HDL-C concentration >7.5 mmol/l |
| CVD=cardiovascular disease; non-HDL-C=non-high-density lipoprotein cholesterol | |
Statin Intolerance
- Statin intolerance is defined as the presence of clinically significant adverse effects from statin therapy that are considered to represent an unacceptable risk to the patient or that may result in adherence to therapy being compromised
- For people who are intolerant of the recommended statin treatment, see the NHS England Accelerated Access Collaborative statin intolerance algorithm.
References
References

