Background
In recent years, international consensus reports and guidelines have taken into consideration the results of cardiovascular (CV) outcome trials demonstrating CV benefit with sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs)[3–18]
During the development of this algorithm, NICE also updated the treatment algorithm for managing patients in the guideline on management of type 2 diabetes mellitus in adults (NICE guideline 28) to take these CV outcome trials into account[19]
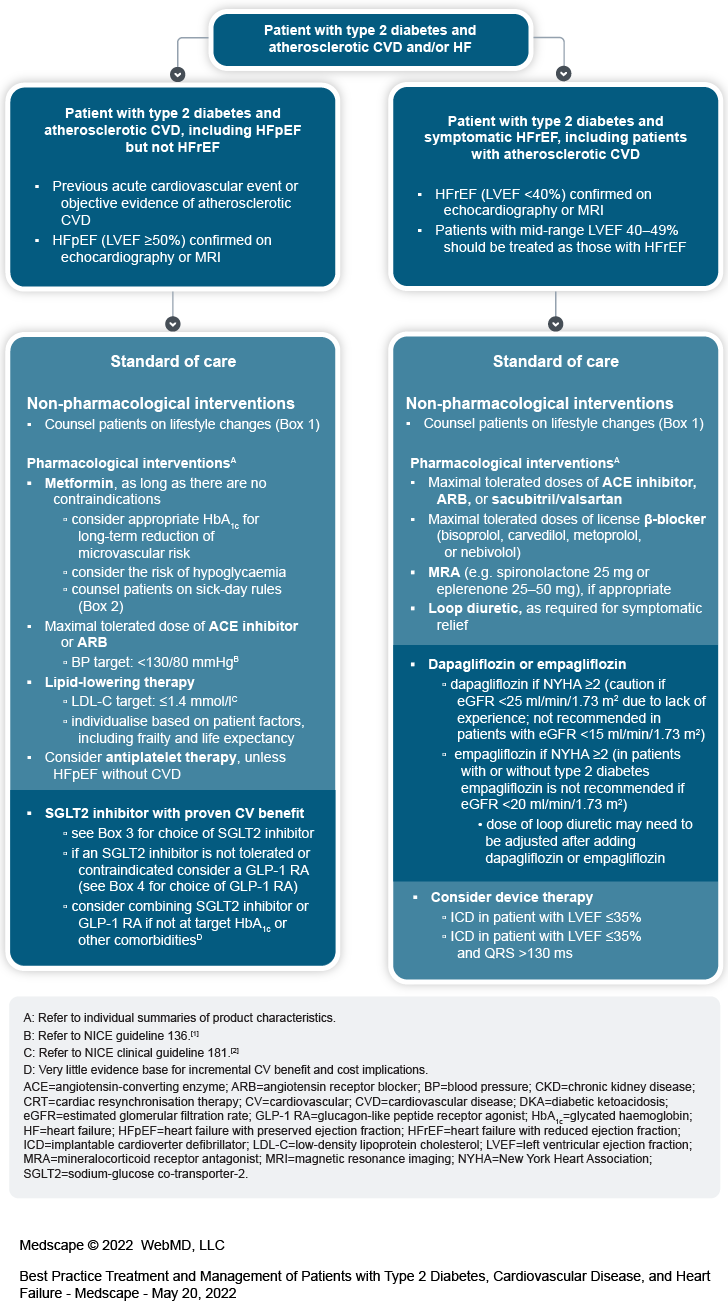
An expert group has developed a consensus treatment algorithm to help UK primary healthcare professionals manage patients with type 2 diabetes, cardiovascular disease (CVD), and heart failure (HF), incorporating the evidence for CV benefit with SGLT2 inhibitors and GLP-1 RAs
The algorithm includes two separate pathways for:
patients with type 2 diabetes and atherosclerotic CVD or HF with preserved ejection fraction (HFpEF) but not HF with reduced ejection fraction (HFrEF)
patients with type 2 diabetes and symptomatic HFrEF, including patients with atherosclerotic CVD
Atherosclerotic CVD includes patients who have experienced a previous acute cardiovascular event for example, acute coronary syndrome, stroke, or transient ischaemic attack or patients with objective evidence of atherosclerotic CVD (for example, stable angina, previous percutaneous coronary intervention, or peripheral arterial disease)
HF should be confirmed by echocardiography or magnetic resonance imaging
HFpEF is defined as left ventricular ejection fraction (LVEF) ≥50%
HFrEF is defined as LVEF <40%
patients with mid-range LVEF 40–49% should be treated as those with HFrEF.
Patients with type 2 diabetes and atherosclerotic CVD in the absence of HFrEF
The priority for treatment of patients with type 2 diabetes, atherosclerotic CVD, and HFpEF but not HFrEF is to treat the diabetes and manage cardiovascular risk:
standard of care includes non-pharmacological and pharmacological interventions
Counsel patients on non-pharmacological lifestyle interventions (Box 1)
| Box 1: Lifestyle changes |
|---|
|
Pharmacological interventions
Prescribers should refer to the individual summaries of product characteristics for further information and recommendations regarding the use of pharmacological therapies
Metformin, as long as there are no contraindications
consider appropriate glycated haemoglobin (HbA1c) for long-term reduction of microvascular risk
take into account the risk of hypoglycaemia
counsel patients on sick-day rules (Box 2)
| Box 2: Sick-day rules |
|---|
|
Maximal tolerated dose of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB)
aim for blood pressure (BP) <130/80 mmHg (refer to NICE guideline 136[1])
Lipid-lowering drugs
aim for low-density lipoprotein cholesterol (LDL-C) ≤1.4 mmol/l
individualise targets for patient factors, including frailty and life expectancy
refer to NICE clinical guideline 181[2]
Consider antiplatelet therapy depending on bleeding risk and severity of atherosclerotic CVD (for example, stenosis >50%), unless the patient is on this pathway because they have HFpEF without atherosclerotic CVD
SGLT2 inhibitor with proven CV benefit
see Box 3 for recommendations for choice of SGLT2 inhibitor
if an SGLT2 inhibitor is not tolerated or contraindicated consider a GLP-1 RA (see Box 4 for recommendations for choice of GLP-1 RA)
consider combining SGLT2 inhibitor or GLP-1 RA if not at target HbA1c or the patient has other comorbidities
be aware that there is very little evidence for incremental CV benefit and there are cost implications
Box 5 provides considerations when using an SGLT2 inhibitor or GLP-1 RA in patients on existing type 2 diabetes therapy.
| Box 3: Recommended SGLT2 inhibitors for patients with type 2 diabetes, atherosclerotic CVD, and HFpEF (but not HFrEF) |
|---|
|
| Box 4: Recommended GLP-1 RAs for patients with type 2 diabetes, atherosclerotic CVD, and HFpEF (but not HFrEF) |
|---|
Liraglutide
|
| Box 5: Considerations when using SGLT2 inhibitors or GLP-1 RAs in patients on pre-existing diabetes therapy |
|---|
CKD=chronic kidney disease; DKA=diabetic ketoacidosis; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide 1 receptor agonist; HF=heart failure; SGLT2=sodium-glucose co-transporter-2; SU=sulfonylurea. |
Patients with type 2 diabetes and symptomatic HF, including atherosclerotic CVD
The priority for treatment of patients with type 2 diabetes and HFrEF is to manage the HF:
standard of care includes non-pharmacological and pharmacological interventions
Counsel patients on non-pharmacological lifestyle interventions (Box 1).
Pharmacological interventions
Prescribers should refer to the individual summaries of product characteristics for further information and recommendations regarding the use of pharmacological therapies
Maximal tolerated doses of ACE inhibitor, ARB, or sacubitril/valsartan
Maximal tolerated doses of licensed β-blocker (bisoprolol, carvedilol, metoprolol, or nebivolol)
Mineralocorticoid receptor antagonist (MRA) (for example, spironolactone 25 mg or eplerenone 25–50 mg), if appropriate
Loop diuretic, as required for symptomatic relief
Dapagliflozin or empagliflozin
dapagliflozin if New York Heart Association (NYHA) class remains ≥2 irrespective of eGFR (caution if eGFR <25 ml/min/1.73 m2 due to lack of experience in this population, not recommended in patients with eGFR <15 ml/min/1.73 m2)[26]
empagliflozin if NYHA remains 2 or more (in patients with or without type 2 diabetes empagliflozin is not recommended if eGFR <20 ml/min/1.73 m2)
dose of loop diuretic may need to be adjusted after adding dapagliflozin or empagliflozin
Box 6 provides considerations when using dapagliflozin or empagliflozin in patients on pre-existing diabetes therapy.
| Box 6: Considerations when using dapagliflozin or empagliflozin in patients on pre-existing diabetes therapy |
|---|
|
Consider device therapy
Implantable cardioverter defibrillator (ICD) in patients with LVEF ≤35%
Cardiac resynchronisation therapy (CRT) in patients with LVEF ≤35% and QRS >130 ms.
Useful resources
British Cardiovascular Society CaReMe resources: www.britishcardiovascularsociety.org/resources/bcs-videos-and-webcasts/careme
Down S. How to advise on sick day rules: diabetesonthenet.com/diabetes-primary-care/how-to-advise-on-sick-day-rules/
IDF. How to manage diabetes during an illness: “sick day rules”: www.idf.org/component/attachments/?task=download&id=2155:IDFE-Sick-day-management
NICE. Cardiovascular disease: risk assessment and reduction, including lipid modification: www.nice.org.uk/cg181
NICE. Hypertension in adults: diagnosis and management: www.nice.org.uk/ng136
NICE. Type 2 diabetes in adults: management: www.nice.org.uk/ng28
Think Kidneys “sick day” guidance: www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf
Date of preparation: April 2022
Guidelines identified a need for clinical guidance in a specific area and approached AstraZeneca for an educational grant to support the development of a management algorithm. The grant included honoraria for the contributors. This algorithm was developed by Guidelines, and the Chair and members of the working group were chosen and convened by Guidelines. The content is independent of and not influenced by AstraZeneca, who checked the final document for technical accuracy only. The views and opinions of the contributors are not necessarily those of AstraZeneca, or of Guidelines, its publisher, advisers, or advertisers. No part of this publication may be reproduced in any form without the permission of the publisher.
Conflicts of interest: The group members have received an honorarium to develop this algorithm. Some of the group members have also received consultancy fees from other pharmaceutical companies, which may include AstraZeneca, for activities other than the development of this algorithm.
Download
Credits:
Lead Image: andriano_cz/stock.adobe.com
Medscape © 2022 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Best Practice Treatment and Management of Patients with Type 2 Diabetes, Cardiovascular Disease, and Heart Failure - Medscape - May 20, 2022.





Comments